Were magnetic materials useful in cancer therapy?
Abstract
Cancer is one of the major challenges fronting the biomedical basic researches in our time. The study and development of effective therapeutic strategies for cancer therapy are vital. Among the many probable core constituents of nanoparticles, magnetite-based nanoparticles have been widely studied for cancer therapy owing to their inherent magnetic features, multifunctional design, biodegradable and biocompatible properties. Magnetic nanoparticles have been also designed for utilizing as contrast enhancer agents for magnetic resonance imaging, drug delivery systems, and most recently as a therapeutic element in inducing cellular death in tumor ablation therapies. This review aimed to provide an overview of the various applications of magnetic nanoparticles and recent achievements in developing these advanced materials for cancer therapy.
Keywords
1. Introduction
The advent of nanoscale science and technology has been extensively touted as an exhaustive paradigm shift for cancer diagnosis and treatment. Certainly, the increase in research works investigating the synthesis of nanoparticulated systems has done the formation of various material formulations showing hopeful diagnostic and therapeutic effects for the treatment of many types of cancer in a single nano-drug [1], [2], [3]. Nanoparticle configurations consist of those with main cores of organic molecules, inorganic molecules, or a combination of two or more of these elements [4], [5], [6]. All basic structures have some advantages and disadvantages that hinge on the intended use. Moreover, these formulations have properties for example surface charge, hydrophobicity, and size that are tunable, permitting them to be improved for a favorite function [7], [8].
Magnetic nanoparticles (MNPs) are a major group of nanoparticles (NPs) that are usually constructed from pure metals ions or a mixture of polymers [9], [10]. Exosomes, naturally formed NPs, are secreted endogenously from cells upon fusion of an intermediate endocytic compartment, and they have been identified as outstanding vehicles for gene and drug delivery [11]. Several studies have reported that exosomes may be isolated from cell culture media, particularly tumor cells culture [12]. Nevertheless, these cells secrete few exosomes and can offer cancer- and immune-stimulating functions due to their nucleic acids and protein cargoes from donor cells [13], [14]. Recently, an exosome-based superparamagnetic iron oxide nanoparticles (SPIONs) cluster as a targeted drug-delivery vehicle for the treatment of cancer was developed. It was revealed that exosome-based drug delivery increased cancer-targeting under an external magnetic field and repressed tumor [11].
The MNPs have many biomedical applications, such as controlled drug release [15], biosensing [16], hyperthermia cancer treatment [17], magnetic resonance imaging (MRI) [18]. Another application of MNPs is magnetic particle imaging (MPI). MPI is a new imaging technique with the ability to the detection of cancerous cells in different tissues. NPs accumulations in cancerous tissues in comparison to other sites can detect cancerous tissues. This ability is dependent on to shape and physical or chemical characteristics of NPs and their signal generation. The biodistribution of magnetic particles can be evaluated by single-photon emission and near-infrared fluorescence. Iron oxide particles are efficient materials for imaging with high sensitivity and the polymers are compatible and degradable in the body [19]. The main advantage of MNPs is their capability to be magnetically manipulated with an external magnetic field. Morphology, chemical composition, shape, size, and magnetic behavior of the MNPs are the most significant criteria in the determination of their biomedical uses [10]. As well, the magnetic properties and effectiveness of MNPs in vivo might be tailored using a biocompatible and safe coating to enhance their suitability for a specific target in the human body [20]. This coating can form various structures for targeting specific cells or molecules and is used for imaging these. Surface modification approaches can lead to the multifunctionality of MNPs [21], [22]. As chemical modification offers multiplexed functionality for example combined hyperthermia drug delivery and multimodal imaging [20], [23], [24].
It has been revealed that fever could negatively affect cancer cells growth [25]. Besides, cancerous and non-cancerous cells show diverse behavior over a temperature range from 42 °C to 45 °C. Non-cancerous cells could endure these temperatures for a short time whereas cancerous cells undertake apoptosis [26]. Thus, keeping the temperature of the cellular environment in hyperthermia might be an effective way in cancer therapy with lower side effects [27]. MNPs have a large potential for creating heat under magnetic fields and enhancing the cancerous tumor temperature to 42–45 °C [28]. Hence, hyperthermia is currently utilized, in the treatment of many cancers, i.e. uterine, prostate, and breast cancers [29], [30], [31], [32].
Another application of MNPs is in MRI for improving tissue targeting [33]. They could be localized into the tissue sites to enhance proton relaxation and to increase their visibility [34]. Considering as a next-generation material in MRI, MNPs are utilized for imaging contain nanocrystalline particles which might be more functionalized with coating materials and functional ligands. Further precise and efficient measurement of cells and biomarkers is critical in fast diagnosis and prevention of metastasis. Early diagnosis of cancer can considerably avoid the growth of cancer and its metastasis to another tissue [34]. Assessment tools of cancer with large sensitivity in detecting some different targeting moieties that need minimal sample preparation to promote considerably fast diagnosis. The use of MNPs in biosensors has been widely researched. These systems propose unique advantages over conventional detection approaches [3], [35].
This review is dedicated to discussing recent advances in employing magnetic-based materials including superparamagnetic iron oxide materials, silica-based magnetic materials, magnetic gold materials, magnetic chitosan-based materials, magnetic metal-based materials, magnetic carbon-based materials, magnetic polyethylene glycol (PEG)-modified materials, biocompatible polymers, and other magnetic material for monitoring and diagnosing cancer via imaging modalities, the cancer therapy through transportation of biotherapeutic and chemotherapeutic agents, photothermal therapies and magnetic hyperthermia.
2. Applications of advanced magnetic materials for cancer therapy
The use of magnetic-based materials in biomedical applications holds great potential in the field of cancer therapy. Especially, there are numerous properties of magnetic-based nanomaterials including large surface area, and unique optical properties that allow the development of new cancer treatment assays [36]. We will review these material-dependent characteristics of NPs concerning their effectiveness in cancer therapy through hyperthermia, drug delivery, etc.
2.1. Superparamagnetic iron oxide nanoparticles-based materials for cancer therapy
Superparamagnetic iron oxide NPs (SPIONs) are auspicious drug carrier platforms due to their high biocompatibility, T1 and T2 contrast effects for MRI, flexible surface functionalities, and stable colloidal suspensions in vivo [37], [38], [39]. Moreover, SPIONs may be metabolized and biodegraded while not accumulating in the body [34]. The US Food and Drug Administration (FDA) provided several types of SPIONs for clinical utilization [40]. Recent examples of SPIONs based materials for cancer therapy are reviewed below.
Zuvin et al. [41] demonstrated the anti-cancer effects of polyacrylic acid-coated SPIONs on breast tumor cells at the low magnetic field strength of 0.8 kAm-1 by attachment of anti-HER2 antibody to NPs. The modified NPs were successfully internalized by MDA-MB-453 and HER2-positive SKBR3 cell lines. The HER2 overexpressing cancer cells were targeted and then killed by an anti-HER2 antibody conjugated SPIONs by hyperthermia made through induction under lower magnitudes and magnetic field strengths. The modified NPs also showed low toxicity to the cell lines resulted in a noticeable reduction in survival in MDA-MB-453 cells and cell proliferation exposing them to hyperthermia [41]. Compared with other therapeutic methods, this method has increased specificity, consequently, it can also provide a more effective treatment. Furthermore, several features such as high stability, ultra-small size, and resistance to aggregation, make it more appropriate for further in vivo studies.
Magnetically-guided NPs are promising candidates in targeted drug delivery systems. To transport bare MNPs or loaded with drugs to a targeted area, a constant external magnetic field is needed [42]. SPIONs are one of the best options which have acquired increasing attention for their applications in cancer therapy owing to their outstanding stability, good biodegradability, low toxicity, and superparamagnetism [43], [44]. Magnetically-guided drug delivery system is mainly simplified with the developing multifunctional NPs by encapsulation of SPIONs and the chemotherapeutic agents inside the desired polymeric systems. These multifunctional NPs can deliver the agents in a targeted manner within the tumors. To date, the studies reported on the multifunctional MNPs have not fully investigated their physicochemical properties, including cytotoxicity, solubility, magnetic properties, etc. A new multifunctional magnetic-polymeric NPs (MF-MPNs) was explained by Kandasamy et al. [45] based on ferrofluids by individual encapsulation of oleylamine-coated hydrophobic SPIONs into the poly(lactic-co-glycolic acid) (PLGA) NPs, as well as two drugs, curcumin (Cur) or verapamil (Ver). They investigated the magnetic properties, dispersibility, biocompatibility, and heating efficacies of the selected MF-MPNs. Ultimately, the therapeutic efficiency of the MF-MPNs was validated in the treatment of HepG2 liver cancer through two different mechanisms, thermotherapy via using SPIONs and chemotherapy via Cur or Ver. All results revealed that dual-drugs and SPIONs-PLGA NPs improved therapeutic efficiency in HepG2 tumor cells through integrated treatment (chemotherapy and thermotherapy) in comparison to the individual treatment. Hence, the MF-MPNs-based ferrofluids (SPIONs/dual-drugs co-loaded PLGA NPs) showed possible therapeutic options for in vitro multimodal cancer therapy. It has also been shown that PLGA encapsulation could improve the stability of SPIONs without altering or affecting the photothermal potency which can minimize the toxicity and maximize the treatment efficiency of the formulation [46], [47].
Magnetic hyperthermia cancer therapy is another conventional treatment option like chemotherapy and radiotherapy [48]. Interestingly, it has targeting capacity and low systemic toxicity. Nevertheless, this approach has major problems in clinical translation such as agglomeration susceptibility of the nano heating agents in aqueous media, uncontrolled heat generation and dissipation at the target region, and eschewed clearance by reticuloendothelial systems. In this regard, SPION as a potential targeted nano heating agent stabilized by a micellar conformation was fabricated for novel therapeutic applications. It was covered with a thin layer of polycaprolactone (PCL) to obtain higher thermo-sensitivity and cytocompatibility. Based on the in vitro findings, the human liver tumor cells’ viability at the greatest concentration of SPIONs (100 μg/mL) was considerably diminished to 40.1 ± 0.9% in the secure hyperthermia temperature range. This range was obtained with the heating effects of polymer-coated SPIONs in the existence of alternating magnetic field (AMF), representing them on or off cytotoxicity occurrence of polymer-covered SPIONs while exposing them to AMF [49]. The advantages of PCL-coated SPION include structural stability, better dispersity, cytocompatibility, and controlled heating under hyperthermia conditions that introduce it as a promising strategy for active targeting of the carcinoma cells in the future [49].
The synthesis process of iron oxie NPs (IONs) has been assessed in various studies utilizing iron (II) precursors. Nonetheless, the synthesis procedures include the use of different alkaline reagents or bicarbonate solutions, which are not desirable for bio-medical aims [50], [51]. The impacts of different oxidative circumstances were assessed on the magnetic and physicochemical features and cytocompatibility of synthesized SPIONs utilizing a single alkaline reagent and iron (II) precursor. For the first time, they assessed the synthesis of SPIONs through a sole precursor. The synthesis conditions were optimized where the N2:O2 flow ratio playing a critical role in targeting practical uses for cancer therapy. Various dose-response features of the synthesized SPIONs on several alternating magnetic fields (AMF) strengths were also investigated. Then, the induction heating efficacy of the optimum SPIONs was assessed under different AMF exposure. The cell viability was reduced to 49 ± 0.3% by the cytotoxic activity of the synthesized SPIONs on the human liver cancer cells under hyperthermia conditions. The findings revealed that such magnetic nano heating agents are effective candidates for cancer therapy objectives.
Antibody-conjugated NPs could maintain the chemical structure of the drugs and deliver them in a controlled way with low toxicity. Since some breast cancer indications have restricted therapeutic options, this field offers hope for the future treatment of breast cancer patients. Nevertheless, cross-linking of antibodies is not selective and causes the major drawback of lacking control on antibody orientation onto the surface of NPs [52]. In a recently published study, the theranostic ability of SPIONs was assessed to diagnose and treat cancer by establishing a single integrated nanoprobe. Oleylamin-coated SPIONs (SPION-Ol) were synthesized and modified with trastuzumab (TZ) and protoporphyrin (PP). The photothermal ablation and the relaxivity r2 values were determined along with in vitro laser irradiation and MRI. There was no cytotoxicity after incubation of MCF-7 cells under different concentrations of theranostic agents and SPION-Ol. The results showed that water-soluble SPION-PP-TZ is an auspicious bimodal agent for diagnosing and treating human epidermal growth factor receptor (EGFR) 2-positive breast cancer cells utilizing photothermal therapy and a T2 MRI contrast agent [53].
A formulation of SPIONs with polyaspartamide (PA) biopolymers was developed for the treatment of tumor cells through the hyperthermia method. PA is a biodegradable and biocompatible polymer with a polysuccinimide backbone that has a critical role in the encapsulation of SPIONs. Multifunctional drug carriers might be conjugated with further groups like biotin to increase the uptake ability of SPIONs to tumor-cell receptors. The findings revealed that great biocompatible performance is obtained by encapsulation of SPIONs nano heaters with PA biopolymer in terms of cell viability and represented useful cancer-killing activities in both in vivo and in vitro hyperthermia tests. PA-encapsulated SPIONs exhibited good heating ability via hyperthermia experiment in room-condition, increased cancer cellular uptake capability, and high cytotoxicity against cancer cells. These results will open the further potential for cancer treatment in the future [54].
Previous studies have shown that a particular bioactive moiety should be installed on SPIONs for treating breast cancer bone metastasis and targeting bone tissues. It was revealed that short-sized acidic peptides comprising repetitive sequences of aspartic acid [55] preferentially bind to hydroxyapatite, as the main element in the organic composition of bone. Pang et al. [56] reported examples of SPIONs-based material for cancer treatment. They utilized bone targeting SPIONs for inhibiting furin to alleviate breast cancer bone metastasis. In both osteoclast function and tumor cell invasion, proprotein convertase furin plays a vital role. The furin inhibitor, a peptide comprising a repetitive sequence of lysine-aspartic acid-glutamic acid, was delivered via the bone targeting SPIONs for enhancing the circulation of NPs within the bloodstream [56]. Besides, a matrix metallopeptidases (MMPs) 2/9-responsive linker has further attached to furin inhibitory peptide for enhancing the specificity of NPs [57], [58]. The MMP2/9 is an enzyme-responsive stimulus activated with metastatic/invasive phenotypes, which is a crucial element of tumor progression. Simultaneously, MMPs, particularly MMP9, also are critical enzymes in the process of bone resorption. Therefore, a multi-responsive and multifunctional SPIONs system was established. It specifically targets the bone metastatic sites, releases the Furin inhibitory peptide via MMP2/9 prompted cleavage, and generates contrast for the MRI imaging leading to the anti-osteoclastic and theranostic anti-cancer impacts (Fig. 1). The in vivo and in vitro data revealed that such a system can prevent breast cancer invasion and osteoclastic bone resorption resulting in alleviated osteolysis [56].
The peptide–conjugated NPs have shown increased selectivity, greater binding affinity, and more therapeutic efficacy. But, the behaviors of peptide–conjugated NPs in physiological conditions, e. g. intracellular space and bloodstream, have not been understood. Also, the vulnerability of peptides to enzymatic degradation [59], and possible immunogenicity of the engineered peptide–conjugated NPs, and the loss of biological function of the peptides that covalently combined with NPs are some common drawbacks for in vivo and clinical use [60], [61]. From this section, it is concluded that different types of SPIONs-based materials were applied for cancer therapy. An overview of the reported studies that utilized SPIONs-based materials for cancer therapy revealed that combining hyperthermia-targeted SPION approach with dye-labeled anti-HER2 antibody was the efficient approach that effectively achieves tumor specific, local, very effective therapeutic method against breast cancer. Also, the installation of a special bioactive moiety on SPIONs can efficiently be used for targeting breast cancer bone metastasis due to the difficulty of delivering anticancer drugs to the bone. However, inadequate tissue selectivity, poor drug-loading capability, uncontrolled biodistribution of SPIONs are the main drawbacks that limit their clinical translation.
2.2. Silica-based magnetic materials for cancer therapy
Silica-based materials are biocompatible materials with high chemical and physical stability [62], [63]. Recently, several studies have been performed to produce hollow mesoporous silica materials for increasing the efficiency of drug loading [64]. For the production of hollow silica spheres with mesoporous structures, the interior hard and soft templates were eliminated to create the nanocomposite’s hollow structure [65]. Nonetheless, the present synthesis approaches have limitations like time-consuming procedures and complicated experimental setup due to the removing template’s complexity [62], [63], [66]. Hence, it is significant to develop a facile technique for hollow mesoporous silica-based nanocomposite [67]. Here, recent examples of silica-based magnetic materials for cancer therapy are given.
A novel theranostic platform was constructed by Hsiao et al. [68], comprising a drug and a carrier which folic acid-Europium-gadolinium-mesoporous silica was grafted via disulfide bond with L-cysteine (FA-EuGd-MSNs-SS-Cys). It may provide an all-in-one therapeutic and diagnostic instrument with various significant functionalities such as tracing, imaging, and therapeutic drug delivery. To create the carrier (EuGd-MSNs), Europium (Eu3+) and Gadolinium (Gd3+) were doped into MSNs and the conjugation of the Cys on the surface of EuGd-MSNs is performed through disulfide bond linkage [69]. Hence, simple penetration is allowed into the cell while the controlled-release of the drug therapy. Moreover, FA targeting was obtained more efficiently with phagocytosis specificity and selectivity. The findings revealed that multifunctional theranostic FA-EuGd-MSNs-SS-Cys can be effective nanoplatforms for imaging-guided cancer treatment and simultaneous therapeutic agents. Evaluating the cellular uptake indicated that more cancer cells are trapped by FA-EuGd-MSNs compared to EuGd-MSNs. The cell materials revealed that Eu3+ emitted the intense red fluorescence, a considerable quantity of FA-EuGd-MSNs is swallowed with the Hela cells. Also, the photoluminescence sensitivity is enhanced by the paramagnetic functionality of the Gd3+ through MRI. The Cys appeared as an anticancer reagent in a cytotoxicity assessment of FA-EuGd-MSNs-SS-Cys using 2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Excellent features were also revealed by EuGd-MSNs such as the nonexistence of cytotoxicity, stability, and decent biocompatibility when cultivating with normal L929 cells [68]. Advantageously, this approach can operate as a useful nano platform for a simultaneous therapeutic agent and imaging-guided treatment for cancer. Simultaneous diagnosis and treatment in this research are an important benefit over previous studies.
MNPs-based hyperthermia therapy has a variety of benefits comparing conventional hyperthermia therapy including targeted delivery resulting in more selective and effective treatment, localized hyperthermia, efficient blood-brain barrier (BBB) crossing, reduction of the needed effective dose of toxic chemotherapeutic drugs, etc. [70], [71], [72]. In this regard, a magnetic with potential chemo- hyperthermia therapeutic properties was established in terms of thermo-responsive copolymer-coated magnetic mesoporous silica NPs (MMSNs) with poly(N-isopropyl acrylamide-co-methacrylic acid) (P(NIPAM-co-MAA)). Loading of the model anti-cancer drug, doxorubicin hydrochloride (DOX.HCl), was performed over MMSNs@P(NIPAM-co-MAA). Temperature- and pH-responsive drug release performance was exhibited by DOX-MMSNs@P(NIPAM-co-MAA) and MMSNs@P(NIPAM-co-MAA). The DOX release was accelerated by a lower pH environment and hyperthermia temperature. The results of cell culture revealed hyperthermia, leading to a higher efficacy for killing cancer cells. Hence, MMSNs@P(NIPAM-co-MAA) present an auspicious platform for cancer treatment.
In another study, a tumor microenvironment-responsive biodegradable MnSiO3@Fe3O4 nanoplatforms was provided for dual-mode MRI-oriented combinatorial cancer treatment. Anti-cancer drugs were loaded efficiently into the nanoplatforms in which Fe3O4 NPs block the pores of MnSiO3 NPs, reducing the drug leakage under normal physiological circumstances. Under high-concentration of glutathione (GSH) or weakly acidic conditions, the nanoplatforms were broken leading to the Fe3O4 NPs separation and fast release of the Mn2+ ions and drug. Besides, the specific surface area of magnetic particles can be enhanced by the exfoliated Fe3O4 nanocrystals while improving the catalytic activity of the Fenton-type reaction into the cancer cells. Hence, more OH is generated enhancing the apoptosis of HeLa cells. Based on the in vitro and in vivo tests, outstanding improvement was obtained on the dual-mode MRI contrast and anti-cancer activity of the produced nanoplatform along with decreased systemic toxicity. Also, paramagnetic manganese (Mn2+) might play a role in reducing disturbance and T1 and T2 relaxation times at the same time. Such a multifunctional nanoplatform could efficiently decrease the adverse effects of the drug and might be useful to the real-time monitoring of drug release. Hence, this can be an auspicious option for synergistic cancer therapy and pH-responsive MRI in clinical uses [73].
For effective treatment of tumor-based bone defects, it is yet a big challenge to design and construct multifunctional biomaterials. Both chitosan (CS) and bioglass (BGs) were extensively found for bone repair use owing to their brilliant osteoconductivity, proper biodegradability, and good biocompatibility. SrFe12O19 (strontium hexagonal ferrites) as a MNPs was modified with mesoporous CS/BG porous scaffold and illustrated antitumor function and bone regeneration. It was found that the stem cell osteogenic differentiation and regeneration of new bone were considerably promoted by magnetic SrFe12O19 NPs in modified-mesoporous bioglass (BG)/CS porous scaffold (MBCS) through the activated BMP-2/Smad/Runx2 pathway. Furthermore, it presented perfect photothermal agents for killing residual cancer cells via NIR photothermal treatment. By irradiating near-infrared (NIR) laser, tumor apoptosis and ablation were triggered by the raised temperatures of tumors cocultured with MBCS. Compared to the pure scaffold group, the excellent antitumor efficacy was shown by MBCS/NIR group against osteosarcoma hyperthermia ablation. Advantageously, the multifunctional MBCS with superior photothermal therapy functions and bone regeneration can be effectively used for treating tumor-based bone defects [74].
With current progress in nanocarriers synthesis, it is allowed to use dual-drug delivery systems for encapsulating two drugs and improve the treatment impacts. In this regard, the Fe3O4@SiO2@tannic acid NPs were used as a pH-responsive drug delivery system for simultaneous delivery of methotrexate (MTX) and DOX anticancer drugs. Studies were performed on in vitro drug release and loading performances. Loaded MTX and DOX nano-carriers exhibited pH-controlled-release of drugs in a sustained mode. According to the cytotoxicity study of blank nano-carrier versus MCF-7 cell lines possesses a cytocompatible future. Nevertheless, the co-administrating DOX with MTX possessed considerable cytotoxicity to the MCF-7 cell lines as a result of the pseudo peptide skeletons formation in the nanocarrier. Minimal drug leakage was shown by in vitro drug release performance at pH 7.4 from the Fe3O4@SiO2 @Tann. This reduces the adverse impacts on the normal tissues. So, drug release is considerably higher at pH 5 enhancing the cytotoxicity for tumor tissues. Moreover, the MTT test revealed that the MTX-DOX-loaded nanocarrier possessed greater cytotoxicity against MCF-7 cells comparing the free drugs. The findings revealed that the Fe3O4@SiO2@Tann could be used as a potential targeted drug delivery system to cancer tissues. Moreover, the potential for anticancer treatment was found by the prepared dual anti-cancer drug-loaded pH-responsive dual core-shell MNPs [75]. The advantages of this approach include biocompatibility, thermal stability, rich variety, and easy control of morphology, structure, and size. Also, the simultaneous use of two drugs can greatly enhance their therapeutic effect.
Presently, a huge deal of interest has been attracted by the bimodal photoluminescence-MRI method as a result of its greater potential in clinical practices and biomedical researches. A new tumor-targetable bimodal nano-probe, FA-Gd-Tb@SiO2 was made by Song et al. through covalent binding of a luminescent Tb3+ complex, N, N, N, N-(4 -phenyl-2,2:6,2 -terpyridine-6,6 -diyl) bis (methylenenitrilo) tetraacetate-Tb) into the silica NPs framework, as along with their surface bonding of a tumor-targeting molecule (folic acid, FA) and an MR contrast agent gadoteridol (Gd- DO3A) (Fig. 1). The created nanoprobe displays high r1 and r2 relaxivities, tumor-targeted binding, and stronger long-lived luminescence. Based on in vitro cellular time-gated luminescence (TGL) imaging the FA-Gd-Tb@SiO2 nanoprobe can be identified and accumulated in tumor cells overexpressing the FA receptor. Moreover, in vivo assessment indicated that the as-prepared nano-probe can efficiently improve TGL intensity and T1-weighted MR contrast in cancer tissue. Hence, it contributes to the accurate tracing and detection of tumor cells, and the identification and treatment of tumors clinically. Advantageously, this study suggests a new method for the design of a very targeted, multifunctional, size-controlled nano-drug with an outstanding photothermal effect and excellent pH-responsive performance in drug release and loading [76].

Fig. 1. The synthesis process of bimodal TGL/MR imaging nanoprobe and its use for tracing tumor cells in the tumor-bearing mic. (Reprinted from publication Ref. [76], Copyright (2021),with permission from Elsevier (license code; 5157770388261).
Interesting research was reported for a combination of photothermal and chemotherapy treatment with DOX-loaded Fe3O4@SiO2 nano-drug system. Using a simple solvothermal technique, it was prepared by Fe3O4 MNPs to control the ratio of the reactants for obtaining magnetic Fe3O4 particles with a modifiable size (20∼400 nm). Loading DOX onto the Fe3O4@SiO2 composite particles pH-sensitive and simply release occurred in an acidic setting. This is vital for the particular drug release of tumor cells since the tumor cells possessed acidic internal settings reducing the toxic side impacts on the normal cells. By NIR light (808 nm) irradiating, photothermal therapy was induced by the photothermal effect created by Fe3O4@SiO2@DOX while enhancing drug release. Cellular uptake and cytotoxicity of Fe3O4@SiO2@DOX nano-drugs were assessed in A549 lung tumor cells. Almost 82.8% of A549 lung cancer cells could be killed by treatment with Fe3O4@SiO2@DOX containing only 10 μg/mL of DOX. Moreover, 81.3% of lung carcinoma A549 cells were killed during incubation with Fe3O4@SiO2@DOX that comprises just 0.5 μg/mL of DOX and 15 min of NIR irradiation, thus proposing an exceptional synergistic chemo/photothermal effect in the treatment of cancer [77].
Recently, a facile technique to construct IONP-hollow mesoporous silica spheres (IONP-HMSs) was designed and established through the oil in water microemulsion approach. This drug delivery system has many advantages including simple large-scale generation, adjustable uniform pore size, high surface area, and large pore volumes. In this study, cetyltrimethylammonium bromide (CTAB) encapsulated IONP spheres were provided the template to construct mesoporous silica shells. In the release and loading of DOX in vitro, the IONP-HMSs were utilized as a nanocarrier. Moreover, analysis was performed on the efficacy of DOX-loaded IONP-HMSs for cancer treatment and its biocompatibility through cellular cytotoxicity examinations. The findings revealed that IONP-HMSs possessed a high efficiency of drug loading that allows the pH-trigged release of DOX in vitro. Furthermore, the IONP-HMSs represented outstanding bio-compatibility and increased DOX therapeutic effect to the HeLa cells [67].
2.3. Magnetic gold materials for cancer therapy
Plasmonic NPs like gold are utilized for the controlled-release drugs as a result of their high photo energy conversion into heat [78]. This can activate the preloaded drug release [79]. For cancer therapeutics, multifunctional nanocomposites with interface interactions and controlled structures are attractive carriers [80]. Magnetically and optically activated nanocomposites like dumbbell-like NPs comprising two chemical surfaces which are appropriate for pH-sensitive drug delivery and synchronized cell targeting [81], [82]. Nevertheless, a pH-responsive system might not act well in complex environmental changes when interfering with other factors with the release procedure. In the following, we will review the recently published studies of magnetic gold materials for cancer therapy.
Presently, Jian et al. synthesized positively charged dumbbell-like gold MNPs (Au-Fe3O4 NPs) for targeted VEGF aptamers' delivering into ovarian tumor cells. The dominance interaction between DNA aptamers and targeting VEGF with the surface of Au-Fe3O4 is electrostatic absorption. Confocal microscopy was used to observe the targeted recognization of ovarian tumor cells via the aptamers-functionalized Au-Fe3O4 NPs (Apt-Au-Fe3O4 NPs). It was found that Apt-Au-Fe3O4 NPs specifically binds with SKOV-3 ovarian tumor cells, resulting in the marked intracellular release of aptamers over plasmon resonant light radiation. Hence, the in vitro inhibition is enhanced versus the proliferation of tumor cells. The findings revealed the high potential of Apt-Au-Fe3O4 NPs as a targeted cancer hyperthermia carrier through remote control with high temporal/spatial resolution. The unique features and aptitudes of aptamers include great binding affinity, small size, high specificity, non-immunogenicity, and ease of modification comparing the traditional monoclonal antibodies. The main drawbacks of aptamers for clinical uses contain low selectivity that restricts them in cell targeting and exact release of aptamers. For efficient therapeutic and highly sensitive diagnostics applications, the dumbbell-like Au-Fe3O4 NPs will possess large potential as nano-carriers [84].
The efficiency of photothermal therapy in treating cancer has been demonstrated in former studies. However, it is not probable to assure the selective and complete elimination of all cancer cells, because that numerous cells may escape and causing cancer recurrence [85]. Therefore, combining two or more therapeutic approaches can overcome the severe restrictions encountered by the separate utilization of each treatment [86], [87], [88]. Adjuvant-targeted chemo/photothermal therapy is one of the integrated oncological modalities. This could be applied for drug delivery and incrementing the local temperature at the tumor site while not influencing the normal adjacent tissues [89], [90], [91], [92]. This integrated treatment could be obtained utilizing an all-in-one nanohybrid. It may be triggered using laser beams serving as the external stimulus to induce photothermally and act as drug nanocarriers [93]. Moreover, the thermal effects can increment cellular responses for chemotherapeutic drugs. Hence, this therapy is allowed to administer in lower doses [94], [95]. Recently, further efforts have been assigned to designing potent theranostic NPs and integrate diagnostic and therapeutic agents, for effective cancer therapy. Elbialy et al. established multifunctional magnetic gold NPs (MGNPs) for selective delivery of the drug to the tumor site in a controlled-release mode by utilizing magnetic targeting; stimulate photothermal therapy with generating heat by near-NIR laser absorption, and serve as contrast agents for MRI. The produced MGNPs were characterized via various physical methods. Then, they were conjugated with PEG and DOX to form MGNP-DOX conjugates. The higher efficiency of MGNP-DOX for integrated chemo/photothermal treatment was found both in vivo and in vitro. The immunohistochemical studies and histopathological examination confirmed the efficiency of MGNP-DOX as theranostic material. Furthermore, MGNP-DOX represented decent potential as MRI contrast agents for directed chemo/photothermal synergistic therapies. This work enhances the comprehension of this therapeutic policy, thus optimizing and using MGNPs as the multifunctional drug delivery systems [96]. Also, in another study, a multifunctional drug delivery system has developed based on red blood cells (RBCs) for drug delivery and imaging-guided combination therapy of cancer. MNPs coated with chlorine e6 (Ce6), were attached to the RBCs membrane and then DOX was loaded within the RBCs. Ex vivo and in vivo imaging data showed the very efficient magnetic field-induced tumor homing of those RBCs after intravenous injection into mice [97].
Triple-negative breast cancer (TNBC) is a kind of greatly aggressive cancer, which is difficultly cured via common chemotherapy. The reason is mostly the TNBC drug resistance and lack of efficient tumor-targeting capability of the present drugs [98]. Hence, it is urgently required to develop new approaches for high-efficient and precise TNBCs therapy.
A multifunctional magnetic gold nano-heterostructure was fabricated with photosensitizer Ce6 loading (MF-MGN@Ce6) for synergistic photodynamic and photothermal treatment (PTT/PDT) of TNBC (Fig. 2). The mitochondria-targeting molecular and cell membrane-targeting peptide of cRGD of TPP was functionalized over the nanosystem to obtain MF-MGN@Ce6@RT. Both in vitro and in vivo assessments revealed superb biocompatibility of MF-MGN@Ce6@RT. The findings revealed cRGD/TPP dual targeting design guarantee the accurate delivery of MF-MGN@Ce6@RT to TNBC tumors. It also enhanced considerably the nanosystem’s phototherapeutic effects. The heterostructure of MF-MGN@Ce6@RT integrating both components of gold and iron oxide, MF-MGN@Ce6@RT was revealed as a superior contrast factor for MRI/CT/PA trimodal imaging of in vivo tumors. Hence, it leads to complete tumor growth suppression. Their results, present new nanoplatforms for high-efficient and precise identification and treatment of TNBCs [99].

Fig. 2. Schematic diagrams for fabrication of MF-MGNs@Ce6@RT and its application in multimodal diagnosis, phototherapy combined photothermal and photodynamic treatment. (Reprinted from publication ref. [99], Copyright (2021), with permission From Elsevier (license code; 5157770618319).
2.4. Magnetic chitosan-based for cancer therapy
Recently, the application of polysaccharides has attracted much attention in nanomedicine [100], [101]. Particularly, chitosan (CS) as a natural polysaccharide has been utilized in some medical uses [102], such that the cancer therapies by CS as the drug delivery carrier have been extensively described to be a promising and beneficial method with large flexibility, bio-compatibility, and efficacy [103], [104]. With the advancing polysaccharide-based studies, large advancements have been achieved in the field of targeted drug delivery at the clinical trials as well as research [105], [106]. Here, we will discuss the recent advancement of magnetic CS-based material for cancer therapy.
Shanavas et al. assessed hybrid MNPs with PLGA ‘core’, and surface modified with folate-CS conjugate ‘shell’ as MRI contrast and simultaneous anticancer therapeutic agents. The folate-CS conjugates were prepared utilizing carbodiimide crosslinking chemistry for covering well-packed SPIONs with a further layer of PLGA loaded docetaxel. To better encapsulate, the crystals inside the polymer matrix, an optimal ratio of SPION to PLGA was fixed at 1:10 to provide a considerable magnetization feature to the nanocomposite for use as an MR contrast agent. The cancer drugs release from the CS polymer is carried out by external and internal stimuli (Fig. 3). In this study, at acidic pH, the pH-sensitive CS coating triggered drug release from NPs. The receptor-targeted SPION and docetaxel delivery was provided by biocompatible hybrid NPs for anti-cancer treatment and MRI respectively, as examined in both folate receptor-negative and positive cancer cells. Better cellular uptake was shown by folic acid receptor targeting, hence, cytotoxicity was increased against folate receptor overexpressing KB cells. The enhanced cellular uptake directly increased anticancer effectiveness and decreased the T2 relaxation time of the tumor cell environment. Hence, darker T2-weighted magnetic resonance contrast images were provided in comparison with the control cells. The immense potential of this receptor-targeted bio-compatible hybrid core/shell theranostic agent was found for simultaneous MR imaging and treatment of cancer [107].

Fig. 3. Anticancer drugs release from chitosan nanocarriers in response to internal/external stimulus. (Reprinted from publication ref. [107], Copyright (2021), with permission from Elsevier (code of 5157790417598).
Anti-proliferative and antiangiogenic activity of artemisinin was found as a natural anti-malarial agent in the cancer cells with very lower toxicity to the normal cells. It has limited application in cancer treatment owing to its high lipid solubility, since achieving therapeutic concentration lacking toxicity to the normal cells is difficult [108]. Recently, the natural polymer CS was utilized as the formulating agent for loading artemisinin to MNPs. Uniform size NPs are made by optimization of the formulation circumstances. The particles represented good drug loading capability and an effective drug encapsulation with suitable magnetic features. The efficiency of drug encapsulation was found as 55%−62.5% along with the drug loading capacity of 20%−25%. After 48 h, artemisinin was released (almost 62%−78%) from the artemisinin MNPs. Regarding physiologically acceptable external magnetic fields, an improved accumulation of NPs was shown by fluorescein isothiocyanate (FITC) conjugated artemisinin MNPs within the BALB/c mice model’s 4T1 breast tumor tissues [109]. For higher drug loading, more specific drug-release, and more anticancer activity, the below strategy was applied.
Adimoolam et al. established a formulation including DOX conjugated to MNPs via a pH-sensitive imine linkage with glutaraldehyde as a crosslinker. Using a simple hydrolysis technique, synthesizing various quantities of CS functionalized MNPs was performed in situ at room temperature. For the lysosomes and endosomes pH drug release investigations, the as-synthesized DOX conjugated MNPs were utilized at various pH buffer solutions. Cell viability tests on the SKOV3 and MCF7 cell lines revealed that the MNPs conjugated DOX represented an improved therapeutic impact in comparison to equivalent concentrations of the free drug. Another advantage of MNPs is their decreased toxicity to the normal cells as a result of their targeting ability [110].
The chemical crosslinked interaction between synthetic terephthaloyl diisothiocyanate as a crosslinker and CS polymeric chains was accomplished by Eivazzadeh-Keihan et al. [111]. Three-dimensional (3D) crosslinked CS hydrogel was fabricated with high stability, porosity, and homogeneity and then used as the novel substrate to generate novel magnetic terephthaloyl thiourea crosslinked CS nano-composite. The designed magnetic nano-composite performance was assessed by the magnetic fluid hyperthermia process. The specific absorption rate (66.92 w/g) was defined by the alternation of magnetic field (AMF) with a magnetization value of 78.43 emu/g.
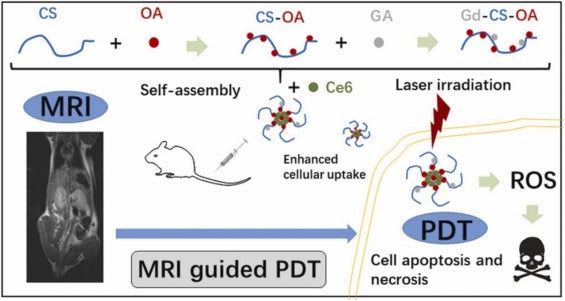
Recently, a CS-derived polymer was successfully produced by Zhao et al. via chemical conjugation of CS, gadopentetic acid (GA), and octadecanoic acid (OA) (Fig. 4). The achieved Gd-CS-OA/Ce6 represented improved MRI ability with desirable biocompatibility and stability. Moreover, the Gd-CS-OA/Ce6 improved the Ce6 uptake into 4 T1 cells both in vitro and in vivo tests. Gd-CS-OA/Ce6 can realize MRI-guided PDT in both tumor-bearing models and 4 T1 cells representing improved anticancer advantages compared to free Ce6. Thus, Gd-CS-OA/Ce6 was indicated as a capable theranostics DDS for efficient cancer treatment [112].

Fig. 4. The figure shows preparation, MRI guided PDT of Gd-CS-OA/Ce6 and drug loading. (Reprinted from publication Ref. [112], Copyright (2021), with permission from Elsevier (license code;5157780028007).
2.5. Magnetic metal-based material for cancer therapy
Metal-based NPs have diverse sizes and shapes and have been studied for their role in the detection and targeted drug delivery [113], [114]. The unique features of metal NPs, for instance, high surface area to volume ratio, facile chemical synthesis, wide optical characteristics, and easy surface functionalization hold promise in the bio-medical field for cancer therapy [115], [116], [117]. These NPs could also be simply functionalized with numerous moieties, e. g. antibodies, peptides, DNA, or RNA to particularly target various cells [118] and with bio-compatible polymers to extend their in vivo circulation for gene and drug delivery uses [119], [120]. Besides, they could effectively convert radiofrequency or light into heat, therefore allowing thermal tumor ablation [121], [122].
Tumor surgical excision is a conventionally therapeutic approach against breast cancer-induced bone metastases; nevertheless, it can cause bone defects and cancer recurrence. For overcoming these problems, Zhao et al. fabricated the multifunctional GdPO4/CS/Fe3O4 scaffolds where the hydrated GdPO4 nanorods were organized in the magnetic bioactive CS matrices for photothermal cancer therapy and bone regeneration. The Fe3O4 NPs in the scaffolds can apply as the photothermal agents for oncotherapy, and the greatly arranged GdPO4·H2O nanorods can promote osteogenesis and angiogenesis abilities. Under the irradiation of NIR laser, local temperatures surrounding the GdPO4/CS/Fe3O4 scaffolds were enhanced to promote tumor cell apoptosis and avoid cancer recurrence. The new blood vessels provided nutrients and oxygen for osteogenesis. Besides, the GdPO4 nanorods in the scaffolds stimulated BMP-2/Smad/RUNX2 signaling pathway that assisted cell proliferation, differentiation, and bone tissue regeneration. Thus, the new GdPO4/CS/Fe3O4 scaffolds with bone defect healing function and photothermal therapy of tumor can become an auspicious platform for the efficient treatment of breast cancer bone metastases [123].
Photosensitizer and nanoparticle conjugates capable of X-ray-induced photodynamic therapy (X-PDT) are a research focus owing to their promising uses in cancer therapy. Jain et al. developed the magnetic-luminescent Gd2.98Ce0.02Al5O12@mSiO2@rose bengal (GAG@mSiO2@RB) nanocomposite to be used as an X-PDT agent. The developed GAG@mSiO2@RB nanocomposite exhibited an effective Fluorescence Resonance Energy Transfer (FRET) upon loading with RB due to great spectral overlap between the RB and GAG. It was found that underexposure with low energy X-rays, the GAG@mSiO2@RB nanocomposite produced 4 times greater singlet oxygen. Effective PDT of MDA-MB-231 cells incubated with GAG@mSiO2@RB nanocomposite was attained upon irradiation with blue light, comparing RB alone. The GAG@mSiO2@RB nanocomposite efficiently generated ROS upon excitation with X-rays. Besides, due to the presence of Gd in the crystal lattice of garnet, the suggested nanocomposite system indicated paramagnetic nature, that can overcome the problems of restricted light penetration for cancer theranostics. Biocompatibility, non-immunogenicity, optimal photoluminescence efficiency, and magnetic properties without any harmful effects in the absence of light, propose GAG@mSiO2@RB nanocomposite as an attractive candidate for its applications. This new magnetic-luminescent nanoplatform is promising for future uses in the simultaneous diagnosis and treatment of deep-lying cancers [124].
Drug delivery systems (DDS) increase the potential of the drug in treatments because of protecting the drugs from fast degrading. Nontoxic MMSN have been applied for the controlled release of drugs and targeting. Fe3O4@mSiO2 NPs were modified with polyethyleneimine linked folic acid (PEI-FA) to enhance their water solubility as well specificity for tumor cells (Fig. 5). Therefore, the cancer-selective disulfiram (DSF) carrier system (mMDPF) was manufactured with an enhanced surface-area-to-volume ratio. The drug loading capability of mMDPF was calculated as 4.35% via HPLC and the best drug release kinetics of mMDPF were detected at pH 6.0 and 37 °C that is the pH in the endosome. The mMDPF cytotoxicity on the MCF-7 cells was enhanced by using mMDPF with sodium or copper nitroprusside. They found that mMDPF was taken up more using MCF-7 cells and its toxicity on MCF-7 cells was much greater than nontumorigenic cells. Advantageously, high solubility and specificity, non-toxicity, selective killing of cancer cells, targeting, and controllable release of drugs are among the benefits that can be mentioned for this drug delivery system [125].

Fig. 5. Schematic diagram of targeted cancer selective therapy with magnetic mesoporous DSF-loaded NPs. (Reprinted from publication Ref. [125], Coppyright (2021),with permission from Elsevier (license code; 5157780228470).
Early-stage detection is crucial for the effective treatment of cancer. Nevertheless, a single mode of MRI is hard to please the high necessities of precise diagnosis due to the innate defects. Jian-Hua et al. constructed a Fe3O4@MnO2@polyacrylic acid NPs (Fe3O4@MnO2@PAA) for the pH-responsive T1/T2 double-model MRI-guided photothermal therapy. The Fe3O4 core provided Fe3O4@MnO2@PAA NPs with the realization capability of T2-weighted magnetic resonance contrast agents, and MnO2 nanoshells proposed a large potential for pH-responsive T1-weighted MRI. This pH-responsive T1/T2 double-model MRI enhanced the specificity and sensitivity and also offered more inclusive data for cancer diagnosis. Additionally, Fe3O4@MnO2@PAA NPs showed great photothermal performance due to the powerful NIR absorption performance, achieving the aim of killing tumor cells without harming the normal cells. Thus, It was revealed that this nanocomposite can be a hopeful pH-responsive theranostic agent for offering accurate and detailed information for the detection and treatment of cancer [126].
2.6. Magnetic carbon-based materials for cancer therapy
In recent years, carbon-based nanomaterials are attracting much attention owing to their capability to operate as a platform for attaching ligands or drugs [127]. Simple models are frequently applied in cancer studies, in which carbon nanomaterials are conjugated to a ligand that is particular to an overexpressed receptor for drug delivery and imaging in cancer therapy [128], [129]. These carbon nanomaterials present unique features to the delivery vehicle or imaging because of their high fluorescence qualities and their nontoxic nature [130]. We look into recently published studies of magnetic carbon-based materials for cancer therapy in the following.
Recently, the therapeutic impacts of NIR irradiation on DOX-resistant A549 cells were assessed and the equivalent in vivo investigations was conducted to assess the behavior of the synthesized substances over targeted chemo/photothermal treatment (chemo-PTT). Aptamer-functionalized Fe3O4@carbon@DOX NPs (Apt-Fe3O4@C@DOX) was reported for synergetic chemo-PTT of cancer (Fig. 6). By the excellent active tumor-targeting ability of Apt-Fe3O4@C@DOX NPs, the simultaneous recognition of target tumors was allowed via MR imaging. Based on in vitro cytotoxicity assay, the integrated chemo-PTT is further toxic to A549 cell lines as compared with PTT or chemotherapy agent alone. The explained therapy reduced tumor growth rates while existing even low quantities of DOX. This is likely caused by intracellular heat generation induced by the photothermal effect. It diminishes the metabolism of DOX and increments DOX accumulation in tumor cells. Such results revealed that the proposed chemo-PTT combination therapy can obtain high therapeutic efficiency against A549-based tumors with outstanding biocompatibility [131].

Fig. 6. Schematic illustration of the preparation of Apt-Fe3O4@C@ DOX NPs and internalization of Apt-Fe3O4@C@DOX NPs into tumor cells for chemoephotothermal combination treatment. (Reprinted from publication Ref. [131], Copyright (2021),with permission from Elsevier (license code; 5157780336267).
It is still challenging to deliver across the BBB and target the brain tumors such as glioblastoma [132]. Hence, novel therapeutic approaches and drug delivery carriers are intensely sought. In this regard, Wu et al. [131] changed the formerly presented concept of particle and made a surfactant-free aqueous ferrofluid. It comprised SPIONs covered with carbon shells and silicate mesolayers. The double covering on SPIONs influenced numerous biological and physicochemical features such as cancer-targeting efficacy and colloidal stability. NPs reduced the viability of osteosarcoma and glioblastoma tumors comparing their non-transformed and primary analogs. A better preference was represented for cancer cells as a result of an enhanced uptake rate of the cells and noticeable adherence to the tumor cell membrane. Sufficient heat was generated by NPs even in an ultralow alternate magnetic field to lead to tumor death. In the basolateral compartments, NPs were found. Moreover, assessing LAMP1 revealed that NPs traversed the BBB transcellular, and escaped the lysosome while localizing to the optic lobes of the third instar larval brains of Drosophila melanogaster. The noninvasive passage resulted in low adverse systemic impacts to the animals. It is deduced that such nanoparticulate ferrofluids attach to cancer cells and, thus, reveal higher toxicity in these cells in comparison to primary cells. Hence, they have higher in vitro effectiveness against solid tumors and can path the BBB in Drosophila. These particles are also non-toxic according to the evolving studies on flies grown in ferrofluid-infused media [131].
There has been a huge deal of interest in new multifunctional core-shell NPs as a result of their abundant functional groups and easy-to-modify surface properties. Recently, Ag@Fe3O4@C-PEG-FA NPs was synthesized with regular structure, uniform size, and higher loading capacities of DOX. Advantageously, the as-synthesized Ag@Fe3O4@C-PEG-FA/DOX NPs had good photothermal impacts and improved DOX release by irradiation of 808 nm light. Furthermore, Ag@Fe3O4@C-PEG-FA/DOX NPs possessed outstanding biocompatibility, stability, synergistic treatment, and cancer cell targeting without any toxic side effects in the treated animals. A superior capacity is provided by multi-armed PEG at the edges of Ag@Fe3O4@C NPs for the materials for loading DOX. As a result of the superior near-infrared (NIR) absorbance capacity of the carbon layer, it can be utilized as a photothermal, Fe3O4 was employed as a reagent for MRI. Multimodal imaging specificities were included for DOX FL imaging and T2-enhanced MR imaging in the tumor area and a decent inhibitory impact on tumor growth synergistic chemo/photothermal treatment. There were no obvious side effects and toxicity in the control and treated animals. Hence, the Ag@Fe3O4@C-PEG-FA NPs are perfect nanoplatforms for chemo/photothermal therapy and multimodel imaging [133].
2.7. Magnetic PEG-modified materials for cancer therapy
PEG-based materials, as a promising candidate for targeted therapy of various cancers, could self-assemble into NPs in the aqueous medium, and offer a stealth surface that might decrease reticuloendothelial system (RES) recognition of NPs and can extend the blood circulation time [134]. In theory, amphipathic PEG-assembled NPs may not be opsonized at all and remain in the blood circulation till penetrates leaky vasculatures in the tumor [118], therefore enhancing the possibility to get their action site. The incorporation of a small part of PEG-based amphiphilic material diminishes RES uptake and opsonization, enhances surface hydrophilicity and the liposome circulation time that contributes to enhancing the concentration of drug in the malignant effusion [135], [136]. So, amphiphilic nanomaterials based on PEG play a key role in passive targeting therapies. Here, we look into recently published studies of magnetic PEG-modified materials for cancer therapy.
A polysuccinimide (PSI) grafted copolymer was synthesized as a cross-linked polymer precursor for the fabrication of biodegradable and biocompatible cross-linked MNPs. The MNPs were covered with the amphiphilic PSI grafted with alkyl and folate-conjugated PEG chains. Over the internal shell of the NPs, the succinimide units were crosslinked and transformed into a biodegradable and biocompatible structure comprising amide bonds. They were more utilized to tolerate free amine groups on the cross-linked MNPs (CMNPs) surface. Ultimately, the CMNPs were conjugated with the near-infrared (NIR) fluorescent dye Cy5.5 for application in particular cancer-targeted magnetic resonance or optical imaging uses. The resultant folate- and Cy5.5- conjugated CMNPs had a diameter of approximately 45 nm, representing brilliant suitability and a higher T2 relaxivity coefficient. Based on the in vivo and in vitro studies, the potential efficacy of CMNPs-Cy5.5-fol is demonstrated as double imaging probes for specific cancer-targeted NIR/MR imaging uses. Advantages of CMNPs include biocompatibility, biodegradability, excellent structural stability in vivo and multifunctionality, specific cancer-targeting, and dual imaging modalities [137].
Presently, monoclonal antibodies are utilized for the treatment of numerous diseases including cancer[138]. Regarding specific tumor-targeted drug delivery, there are numerous efforts to make antibody-conjugated NPs that can show highly improved therapeutic effect in different cancers [139]. Generally, both CD133 and CD44 are accepted as gastric cancer stem cell markers. In a recent study, gastric cancer stem cells were targeted via CD133 and CD44 antibody-conjugated all-trans-retinoic acid-loaded PLGA-lecithin-PEG NPs (ATRA-PLPN) (Fig. 8). Their therapeutic effects were examined against gastric cancer stem cells and the results proved the efficient and special delivery of CD133/CD44 to CD44++ and CD44++ or CD133 gastric cancer stem cells. Hence, growth inhibitory effects were enhanced to gastric cancer stem cells in comparison with single targeted and nontargeted NPs. The present work has reported the elevation of NPs delivery to two groups of gastric cancer stem cells via antibodies. Usually, cancer includes distinct groups of cancer stem cells with many phenotypes. Hence, our dual targeting NPs create efficient drug delivery platforms to target numerous populations of cancer stem cells into cancer. Effective and specific targeting, increased biodistribution and enhanced endocytosis of NPs in tumor cells are some benefits of this drug delivery platform. However, due to being the low number of gastric cancer stem cells in the tumor mass, the optical imaging of the targeting of CD44/CD133-ATRA-PLPN to gastric cancer stem cells in vivo is difficult [140].
Recently, Fe3O4-polymer composite microcapsules with a normal diameter of 885.6 and 587 nm were presented for the magnetic resonance (MR)-guided focused ultrasound surgery (MRgFUS) [141], [142]. They can enhance the energy deposition and ultrasonic wave absorption in the target tissue, hence, the tumor ablative impacts of MRgFUS are increased [141]. The creation of an active targeting PEGylated Fe3O4 NPs platform was demonstrated. Anti-EGFR was used to decorate the surfaces of PEGylated Fe3O4 NPs, for guided delivery to the liver cancer via EGFR overexpression. Furthermore, based on the studies in vitro cellular uptake and cytotoxicity to Hep G2 cells, the EGFR receptor enabled endocytosis can enhance the PEGylated Fe3O4 NPs uptake rate. Thus, the established targeting PEGylated Fe3O4 NPs system might possess the excellent potential for the MRI therapy of liver cancer [143].
IONPs can be collected in the tumor tissues due to their outstanding magnetism. Such particles are T2 contrast agents found via MRI for monitoring its tumor accumulation. Based on previous studies, cancer cells produced hydrogen peroxide (H2O2) at a speed of 0.5 nmol·10 cells each hour [144]. PDT as an FDA-approved cancer therapy modality utilizes non-toxic photosensitizer, O2, and light-generating cytotoxicity ROS mostly O2 for most typical photosensitizers to oxidative damage cancer cells. Nevertheless, the inadequate tumor-targeting capacity of O2 and photosensitizer supply within the solid tumor, the restricted diffusion range of O2, and the short lifetime powerfully limit the efficiency of PDT. For zinc phthalocyanine (ZnPc) delivery, IONPs were loaded within the self-assembly stomatocytes-like structure of poly(ethylene glycol)-block-polystyrene (PEG-b-PS) as nanomotors to transfer photosensitizers to the tumor tissues and enhance PDT efficiency. Under magnetic field, the hybrid nanomotors (iron oxide NPs loaded stomatocytes@ZnPc nanomotors, called as ISP-NMs) could be collected in cancer tissues as a result of IONPs magnetism. After trapping by cancer cells, the decomposition of endogenous H2O2 can be catalyzed by IONPs generating O2 as the propelling force for ISPNMs movement. The distribution of ZnPc was expanded by the motion features of ISP-NMs enlarging ROS reactive distribution and improving the PDT activity. Moreover, the created O2 may be delivered for the PDT procedure ensuring its great performance. Moreover, ISP-NMs possessed a nuclear MRI function because IONPs are effective T2 contrast agents (Fig. 7) [145].

Fig. 7. Schematic illustration of magnetic field assistant tumor targeting of ISP-NMs and their 1O2 production and PDT procedure for cancer therapy. (Reprinted from publication Ref. [145], Copyright (2021),with permission from Elsevier (license code; 5157780493034).
Since an effective strategy is provided by integrating PTT with immunotherapy in the cancer therapy, an MNPs delivery system was made to load immunostimulatory R837 hydrochloride (R837) and indocyanine green (ICG) for spatiotemporally immunotherapy/PTT synergism in cancer. Such a delivery system comprises PEG as the coating layer to load R837 and Fe3O4 MNPs as the core to load ICG. Using intravenous injection, the DPA-PEG coating led to the long circulation, and the superparamagnetism magnetization, PA and PTT function of the Medical Internal Radiation Dose (MIRD) empowered photoacoustic/photothermal multimodal imaging and the magnetic targeting. Here, potent prophylactic and therapeutic impacts with the few side effects were demonstrated systematically. Advantageously, the synergism of the immunotherapy and PTT can prevent, tumor growth, recurrence, and metastasis. Therefore, potent anticancer therapeutic impacts were resultant representing an opening and promising new area for future research [146].
2.8. Biocompatible magnetic polymers for cancer therapy
The development of NPs based on biodegradable and biocompatible polymers for example poly (lactic acid) and poly (glycolic acid), and their copolymers are very interested in researchers. Even with being synthetic, these polymers are degraded in the body into monomers and oligomers that are more removed via the normal metabolic pathways, such as the Krebs cycle [147], [148], [149], [150]. Once polymer NPs are intravenously administered they are subjected to the opsonization reaction, which causes their phagocytosis through the monocyte–macrophages. For overcoming this problem, the particles could be coated with hydrophilic polymers for instance PEG, which avoids recognizing the NPs using the reticuloendothelial system [151], [152]. Recent examples of biocompatible polymers used for cancer therapy are reviewed below.
A new thermo- and pH-sensitive amphiphilic copolymer grafted MNPs was designed and synthesized containing biodegradable and hydrophobic PLA block and hydrophilic P(NIPAAm-co-HEMA-co-MAA-co-TMSPMA) section via a combination of free radical polymerization and ring-opening approaches. Two anticancer drugs MTX and DOX were simultaneously loaded to nano-composite for combination cancer chemotherapy aims. DOX/MTX simultaneous release showed tumor niche (pH≤5.4 and temperature=41 °C) aided release behavior. Cytotoxicity findings disclosed the nontoxicity of newly developed nano-composite to MCF7 cell lines. The anti-tumor feature of DOX/MTX-loaded nano-composite was greater than free drugs discovered by 4',6-diamidino-2-phenylindole (DAPI) staining, MTT assay, RT-PCR, and cell cycle analysis on MCF-7 cell lines. The results exposed that this engineered nano-composite might be successfully utilized in the targeted delivery of MTX and DOX to the tumorous tissues and for more in vivo and clinical applications. Biodegradability, biocompatibility, nontoxicity, targeted drug release, stability in the bloodstream, and proficiency to keep the drug against in vivo decomposition are some benefits of the engineered nano-composite used in this study [153].
In another study, a biocompatible poly-L-lysine-coated MNPs were reported for the combined magnetic resonance imaging and the magnetic hyperthermia to unify the diagnostic and therapeutic method. In this study, biocompatible amino modified MNPs were conjugated to specific antibodies. Then the samples were exposed to calorimetry measurements. According to approximated heating rates the specific absorption rates for the poly-L-lysine modified MNPs (MFPLL) were estimated. Computed specific absorption rate (SAR) values are appropriate for the future study concentrating on the detection of cancer cells mediated with antibodies and on therapy in combination with hyperthermia and MRI. Obtained results showed the important effect of MFPLL on transversal relaxation time T2 with the relaxivity r2 equal to 487.94 mM. It found that combination signifies a substantial advance in cancer disease therapy and a significant enhancement in oncological patients’ survival. The results disclosed specific binding of antibody conjugated MFPLL to carbonic anhydrase IX (CA IX) protein in three-dimensional spheroidal culture. Previous researches have revealed antibody-induced receptor internalization features of VII/20 Mab [154] that may be crucial for the delivery of conjugates into cancer cells and probable usage of this conjugate is combined anti-cancer therapy. The main advantage of MFPLL is selective targeting of colorectal cancer cells that can be important for the possible use of this conjugate combined anticancer therapy [155].
A magnetic pH-responsive natural hydrogel was developed as the effective drug delivery system for the treatment of cancer. Alginate (Alg) was firstly oxidized, afterward, gelatin (Gel) was cross-linked with Alg to yield Alg-Gel chemical hydrogel via a “Shift-Base” condensation reaction. Then, Fe3O4 NPs were integrated into the hydrogel via an in-situ chemical coprecipitation method. The achieved Alg-Gel/Fe3O4 was loaded with DOX, and encapsulation efficiencies, its drug loading, and its anti-cancer activity were studied against Hela cells. In pH = 4 (acidic condition) the Alg-Gel/Fe3O4-DOX showed a greater drug release value than those of the pH 7.4 and 37 °C (physiological condition). It has shown that the constructed magnetic hydrogel has brilliant potential as a drug delivery system (DDS) for the cancer chemotherapy mostly owing to its greater performance than those of the free DOX in terms of pH-dependent drug release behavior, slow drug release profile, magnetic feature for diagnosis through MRI and isolation at the targeted area. Based on the results, the Alg-Gel/Fe3O4 magnetic hydrogel may be studied as a smart DDS for cancer diagnosis and therapy. The advantages of Alg-Gel-DOX and Alg-Gel/ Fe3O4-DOX over free DOX include low toxicity, high biodistribution, slow drug release, and low drug degradation [156].
Zhang et al. successfully developed multifunctional NPs encapsulated with superparamagnetic Fe3O4 NPs. The olaparib (Olb) drug was loaded and the formulation was used for the multimodal treatment of triple-negative breast cancer. Low molecular weight hyaluronic acid (HA) was chosen for coating NPs. Owing to the high affinity between CD44-receptor on the cell surface of triple-negative breast cancer (TNBC) and hyaluronic acid, an effective targeting could be achieved to apply the synergistic antitumor effects both in vivo and in vitro. Using rotating magnetic field (RMF) treatment, HA-Olb-PPMNPs (multifunctional polymeric NPs) generated a physical transfer of mechanical force with an imperfect rotation that the shear forces generated by the rotation, therefore, injured lysosomes and disrupted the cell membrane integrity after HA-Olb-PPMNPs internalized into the cells, by which damaging “two strikes” effects were evident via confocal laser scanning microscopy (CLSM) and scanning electron microscope (SEM) (Fig. 8). So, Olb and the mechanical force apply dual antitumor effect to attain synergistic therapeutic in the presence of RMF. Therefore, magnetic excitation through mechanical force offers an exciting approach to remotely control cell functions for cancer therapy [157].

Fig. 8. Schematic illustration of the cell damage visualized by SEM and TEM. (A) Illustration of the anticancer mechanisms of magneto-cell-apoptosis and magneto-cell-lysis (B) The SEM images of cell membrane integrity damaged using the action of the HA-PPMNPs/HA-Olb-PPMNPs activated by the RMF therapy (red arrow). (C) Illustrative TEM images of magneto-cell-apoptosis triggered by HA-PPMNPs/HA-Olb-PPMNPs under RMF treatment (blue arrow: apoptotic bodies, yellow arrow: intracellular bubbles, and green arrow: karyorrhexis and karyopyknosis. (Reprinted from publication Ref. [157], Cpyright (2021),with permission from Elsevier (license code; 5157780661963).
2.9. Other magnetic materials for cancer therapy
NPs-based theranostics have rapidly emerged in the past decade and have been extensively utilized in the diagnosing and treating of some tumors such as breast cancer and liver cancer. Though, there are limited findings for skin cancers. Hou et al. successfully synthesized multifunctional IR820-grafted CS-Fe3O4 NPs (via grating IR820 onto the surface of CS-coated magnetic iron oxide) for melanoma theranostic uses. This theranostic nanoparticle shows an excellent MRI ability and cytotoxic effects against melanoma under irradiation with a near-infrared laser in vitro. Moreover, they have insignificant cytotoxicity and high stability in the aqueous solution, up to 8 days. Owing to the unique properties, the IR820-CS-Fe3O4 NPs offered a noticeable cytotoxic effect on A375 cells after the irradiation under a NIR laser than conventional PDT. The results showed the good potential of IR820-CS-Fe3O4 NPs for the diagnosis and treatment of melanoma [158].
Biomineralized bacterial magnetic particles (BMPs) have been extensively researched for bio-medical uses with their magnetic features and a layer of bio-membrane. BMPs have been utilized for magnetically targeted drug delivery, magnetic hyperthermia, MR imaging, magnetic detection, and separation [159], [160], [161]. Advantages of the BMPs over MNPs include good dispersion, large production, high crystallinity, and close-to-bulk magnetization [159], [162]. Wang et al. firstly applied BMPs for magnetically targeted photothermal therapy of tumors in vivo. The BMPs were inoculated into the tumor tissue by the self-built C-shaped bipolar constant magnet with a great gradient magnetic field at the tumor site. For the in vitro simulated experiment, BMPs had a great maintenance rate in the magnetically targeted area with diverse flow rates. With the magnetic targeting, the inoculated BMPs with NIR light irradiation for the photothermal therapy can cause a complete tumor eradication. It offers an effective method for the bio-medical uses of BMPs [163].
In recent years, the synergistic combining plasma technology and NPs chemotherapeutic delivery system has shown its capacity in the treatment of cancer [164]. The unique advantages of CAP including sterilization, wood healing, blood coagulation, tooth bleaching, skin regeneration, and cancer therapy have assisted recent biomedical uses [165], [166], [167], [168], [169], [170], [171]. Nevertheless, no report exists on CAP combining iron oxide-based MNPs. The impact of co-treatment of the iron oxide-based MNPs and cold atmospheric plasma (CAP) were reported both in vivo and in vitro for the targeted lung cancer therapy. In this regard, the synergistic impacts of iron oxide-based MNPs and CAP on the expression of EGFR, cellular bioactivity, and EGFR downstream signaling pathways were studied (Fig. 9). The results revealed that the co-treatment of the iron oxide-based MNPs and CAP can efficiently avoid cell proliferation through viability reduction and apoptosis induction. In vivo setting reveals that the CAP combining MNPs is useful at suppressing xenograft tumors. Therefore, the combination of CAP and the iron oxide-based MNPs offers a hopeful tool for developing a novel cancer therapy approach [172].

Fig. 9. Schematic illustration of the molecular mechanisms of MNPs increasing tumor-selective killing impact of CAP. CAP-originated reactive species will result in a significant increase of intracellular H2O2, Fe2+/Fe3+ released from the lysosome comprising MNPs can catalyze H2O2 into OH., which results in the carcinoma cells damage, e. g. making mitochondria-induced apoptosis and double-strand DNA breaks. (Reprinted from Ref. [172], Copyright (2021),with permission from Elsevier (license code; 5157780788522).
Munir et al. for the first time demonstrated that capping agents increase the magnetization power while decreasing the particle size too. In this study, the synthesis of un-capped, and malic acid (MA)-capped and citric acid (CA)-capped iron oxide NPs was done by the coprecipitation approach. For the in vivo bioassay, the capped MNPs were inoculated within the tumor cells followed by exposure to alternating magnetic fields. Generally, the results showed the best suitability of MA and CA-capped iron oxide NPs for treating the mice breast cancer through hyperthermia therapy. Exposure to the alternating magnetic field (AMF) of 15 mT and the frequency of 100 kHz for 1 h resulted in an increase in the temperature (from 37 to 48 oC) and also caused cellular injury and following apoptotic cell death. Overall, functionalized Fe3O4-NPs showed a reduction in particle size, enhanced features and enhanced efficiency for carcinoma therapies by hyperthermia. Hence, the CA-capped iron oxide NPs claims significant outcome that might be in future very assisting for overcoming the enormous casualties owing to cervical/breast cancer.
The developing nano- catalytic therapy can in situ catalyze the endogenous substances into very toxic species and then effectively kill the tumor cells, nevertheless, the lack of high-performance nano-catalysts hampers their wide clinical translation. For the first time, Dai et al. have successfully developed, nano-sized zero valences crystalized iron-polyvinyl pyrrolidone NPs (nZVCI-PVP NPs) for in situ causing nano-catalytic Fenton reaction inside TME to generate large quantities of the hydroxyl radicals and then kill the tumor cells, that might be more synergistically increased by magnetic or photonic hyperthermia as aided with these iron NPs acting as magneto-thermal or photo-thermal conversion nano-agents, respectively (Fig. 10). This study not only offers a new kind of iron-based NPs for bio-medical use but also reveals the high efficacy of nano catalytic cancer treatment as aided by both magnetic and photonic hyperthermia [173].

Fig. 10. The figure shows the nZVCI-PVP for in vivo T2-weighted MR imaging-guided synergistic nanocatalytic treatment and photonic and magnetic hyperthermia of cancer. (a) modification of surface PVP of nZVCI NPs, (b) MR imaging functions of nZVCI-PVP NPs, (c) synergistically increased nanocatalytic treatment and photothermal tumor ablation (d) synergistically increased nanocatalytic tratment and magnetic tumor ablation as aided using nZVCI-PVP NPs. (Reprinted from publication Ref. [173], with permission from Elsevier (license code; 5157780928927).
3. Conclusions and future outlooks
MNPs show great promise for application as an imaging contrast enhancer and therapeutic agent for cancers that are conventionally treated by combining chemotherapy, radiation therapy, and surgical resection. Moreover, the MNPs show great potential for utilization in monitoring cancer treatments and might provide critical data to physicians letting them adapt treatment approaches to offer ideal care. MNPs have seen extensive progress by research teams in the world, still, limited formulations have been approved for clinical applications. Many attempts for developing MNPs for cancer theranostics have continued unabated. A few ongoing clinical studies applying MNPs exist for the hyperthermia therapy of different cancers. Besides, research teams continue to optimize synthesis procedures for the functionalization of MNPs by a biotherapeutic agent, chemotherapeutic, and imaging contrast. Translation of MNPs from lab to clinic needs a whole understanding of the effects which MNPs parameters have on the biological responses of the human. Many MNP formulations have failed to produce acceptable levels of treatment in big animals and humans; like what was observed in small animal models. For enhancing the success rate of MNPs translation into the clinic, attempts must be concentrated on increasing the drug-loading capability, enhancing their affinity and specificity to target tumor cells, and obtaining capacity in microenvironment-dependent drug-release. Moreover, predicting the best formulation of MNPs for humans is achieved with the help of better theoretical animal models. As our comprehending of MNPs nature increases, we are hopeful that combine imaging and multimodal treatment with MNPs will shove forward their clinical uses and significantly affect the treatment of cancers in the near future. Table 1 limits some features of the reported magnetic-based materials for cancer therapy.
Table 1. Summary of the reported magnetic materials for cancer therapy.
| Material | Target | Specific feature | Advantages and/or limitations | Ref. |
|---|---|---|---|---|
| Polyacrylic acid-coated SPIONs | breast tumor cells | anti-HER2 antibody | Advantages: High stability, ultra-small size, and resistance to aggregation | [41] |
| Empty Cell | Limitation: reduce the effect commonly seen on Brownian relaxation-related heating | |||
| SPIONs/Cur/Ver loaded PLGA nanoparticles | HepG2 liver cancer | PLGA encapsulation | Advantages: Improved stability, minimized toxicity and maximized treatment efficiency | [45] |
| PCL-coated SPION | human liver tumor cells | Polymer-covered SPIONs | Advantages: structural stability, better dispersity, and cytocompatibility | [49] |
| TZ-conjugated SPION–porphyrin | MCF 7 | Antibody-conjugated nanoparticles | Advantages: Low cytotoxicity and water-solubility | [53] |
| PA-encapsulated SPIONs | cancer cells | biotin-conjugated | Advantages: good heating ability, and increased cancer cellular uptake capability | [54] |
| Empty Cell | Limitation: high cytotoxicity | |||
| FA-EuGd-MSNs-SS-L-cysteine (Cys) | Hela cells | FA targeting | Advantages: Low cytotoxicity, high stability, simultaneous diagnosis and treatment | [68] |
| MnSiO3@Fe3O4 | HeLa cell | – | Advantages: Decreased toxicity, and real-time monitoring of drug release | [73] |
| FA-Gd-Tb@SiO2 | Tumor cells | – | Advantages: Favorable targeted, multifunctional, size controlled nanodrug, outstanding photothermal effect and excellent pH-responsive performance in drug release and loading | [76] |
| CTAB encapsulated IONP spheres | HeLa cells | – | Advantages: Simple large-scale generation, adjustable uniform pore size, high surface area and large pore volumes | [67] |
| Empty Cell | Limitation: Toxic at the high concentration of CTAB | |||
| Fe3O4@SiO2@DOX nano-drugs | A549 lung tumor cells | – | Advantages: exceptional synergistic chemo/photothermal effect, and high cytotoxicity and cellular uptake | [77] |
| Apt-Au-Fe3O4 | SKOV-3 ovarian tumor cells | aptamers-functionalized | Advantages: great binding affinity, small size, high specificity, and non-immunogenicity | [84] |
| Empty Cell | Limitation: Limited selectivity that restrict them in cell targeting and exact release of aptamers | |||
| MGN@Ce6@RT | TNBC tumors | cRGD/TPP dual targeting | Advantages: Superb biocompatibility, high-efficient and precise identification and treatment of TNBCs | [99] |
| Gd-CS-OA/Ce6 | 4T1 cells | – | Advantages: desirable biocompatibility and stability | [112] |
| GdPO4/CS/Fe3O4 | breast cancer bone metastases | – | Advantages: Increased apoptosis and avoid cancer recurrence | [123] |
| Empty Cell | Limitation: Low water solubility | |||
| GAG@mSiO2@RB | deep lying cancers | – | Advantages: Biocompatibility, non-immunogenicity, and high photoluminescence efficiency | [124] |
| Apt-Fe3O4@C@DOX | A549 cells | aptamer-functionalized | Advantages: Outstanding biocompatibility, high therapeutic efficiency, excellent active tumor-targeting ability | [131] |
| Ag@Fe3O4@C-PEG-FA/DOX | Tumor cells | multi-armed PEG | Advantages: Good photothermal impacts and improved DOX release, outstanding biocompatibility and stability | [133] |
| CD44/CD133-ATRA-PLPN | gastric cancer | antibody-conjugated ATRA-PLPN | Advantages: Effective and specific targeting, increased biodistribution and enhanced endocytosis of NPs in tumor cells | [140] |
| Empty Cell | Limitations: The difficulty of optical imaging due to being low number of gastric cancer stem cells in the tumor mass | |||
| poly-L-lysine-coated magnetic NPs (MFPLL) | colorectal cancer cells | conjugation to specific antibodies | Advantages: Selective targeting | [154] |
| Alg-Gel/Fe3O4-DOX | Hela cells | – | Advantages: Low toxicity, high biodistribution, slow drug release and low drug degradation | [156] |
| HA-Olb-PPMNPs | TNBC | – | Advantages: Effective targeting | [157] |
| IR820-grafted CS-Fe3O4 | Skin melanoma Cell | – | Advantages: Insignificant cytotoxicity and high stability | [158] |
*FA (folic acid).
CRediT authorship contribution statement
Dr. Baghban: Roles/Writing - original draft; Formal analysis; Investigation. Dr. Afarid: Writing - review & editing, Investigation. Dr. Soleymani: Project administration; Supervision; Conceptualization; Investigation; Roles/Writing - original draft; Writing - review & editing. Dr. Rahimi: Project administration; Supervision; Conceptualization; Investigation; Roles/Writing - original draft; Writing - review & editing.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
RB and MA would like to thank supports of Shiraz University of Medical Sciences (Shiraz, Iran) and JS would like to thank support of Tabriz University of Medical Sciences (Tabriz, Iran). MR acknowledge the financial support from National Science Centre, Poland, via Grant UMO-2018/29/B/ST5/02412.
References
-
[1]
Recent progress on nanoparticle-based drug delivery systems for cancer therapyCancer Biol. Med., 14 (3) (2017), pp. 228-241
-
[2]
New insights into designing hybrid nanoparticles for lung cancer: diagnosis and treatmentJ. Control. Release, 295 (2019), pp. 250-267
-
[3]
Magnetic nanoparticles in cancer therapy and diagnosisAdv. Healthc. Mater., 9 (9) (2020), p. 1901058
-
[4]
Multifunctional nanoparticles for brain tumor imaging and therapyAdv. Drug Deliv. Rev., 66 (2014), pp. 42-57
-
[5]
Cancer therapy: cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers (adv. Mater. 36/2011)Adv. Mater., 23 (36) (2011), pp. H209-H309-47
-
[6]
Development and characterization of a novel conductive polyaniline-g-polystyrene/Fe3O4 nanocomposite for the treatment of cancerArtif. Cells Nanomed. Biotechnol., 47 (1) (2019), pp. 873-881
-
[7]
Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advancesMater. Today, 19 (3) (2016), pp. 157-168
-
[8]
PEG-mediated synthesis of highly dispersive multifunctional superparamagnetic nanoparticles: their physicochemical properties and function in vivoACS Nano, 4 (4) (2010), pp. 2402-2410
-
[9]
Iron oxide magnetic nanoparticles supported on amino propyl-functionalized KCC-1 as robust recyclable catalyst for one pot and green synthesis of tetrahydrodipyrazolopyridines and cytotoxicity evaluationAppl. Organomet. Chem., 34 (3) (2020)
-
[10]
Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticlesAdv. Colloid Interface Sci., 281 (2020), Article 102165
-
[11]
Blood exosomes endowed with magnetic and targeting properties for cancer therapyACS Nano, 10 (3) (2016), pp. 3323-3333
-
[12]
Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-primingNat. Med., 7 (3) (2001), pp. 297-303
-
[13]
Tumour-released exosomes and their implications in cancer immunityCell Death Differ., 15 (1) (2008), pp. 80-88
-
[14]
Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic nicheCancer Res., 71 (15) (2011), pp. 5346-5356
-
[15]
Co-delivery of doxorubicin and methotrexate by dendritic chitosan-g-mPEG as a magnetic nanocarrier for multi-drug delivery in combination chemotherapyPolym. Chem., 8 (47) (2017), pp. 7333-7350
-
[16]
Magnetic nanoparticles‐embedded enzyme‐inorganic hybrid nanoflowers with enhanced peroxidase‐like activity and substrate channeling for glucose biosensingAdv. Healthc. Mater., 8 (9) (2019), p. 1801507
-
[17]
Temperature-controlled magnetic nanoparticles hyperthermia inhibits primary tumor growth and metastases disseminationNanomed.: Nanotechnol. Biol. Med., 25 (2020), Article 102171
-
[18]
Magnetic nanoparticles as MRI contrast agentsSurf. -Modif. Nanobiomater. Electrochem. Biomed. Appl. (2020), pp. 49-91
-
[19]
Tomographic magnetic particle imaging of cancer targeted nanoparticlesNanoscale, 9 (47) (2017), pp. 18723-18730
-
[20]
Current trends in chemical modifications of magnetic nanoparticles for targeted drug delivery in cancer chemotherapyDrug Metab. Rev., 52 (1) (2020), pp. 205-224
-
[21]
Surface modification of magnetic iron oxide nanoparticlesNanomaterials, 8 (10) (2018), p. 810
-
[22]
M. Angelakeris, Magnetic nanoparticles: a multifunctional vehicle for modern theranostics, Biochim. Biophys. Acta (BBA)-Gen. Subj. 1861(6) (2017) 1642–1651.
-
[23]
Development of superparamagnetic nanoparticles coated with polyacrylic acid and aluminum hydroxide as an efficient contrast agent for multimodal imagingNanomaterials, 9 (11) (2019), p. 1626
-
[24]
Targeted magnetic nanoparticle hyperthermia for the treatment of oral cancerJ. Oral. Pathol. Med., 48 (9) (2019), pp. 803-809
-
[25]
Effects of local and whole body hyperthermia on immunityHyperth. Cancer Treat.: A Prim. (2006), pp. 247-275
-
[26]
Hyperthermia versus oncothermia: cellular effects in complementary cancer therapyEvid. -Based Complement. Altern. Med., 2013 (2013)) (2013), pp. 1-12
-
[27]
Magnetic nanoparticle hyperthermia, Emerging Electromagnetic Technologies for Brain DiseasesDiagn., Monit. Ther., Springe (2018), pp. 129-191
-
[28]
Chapter 16 - magnetic nanoparticles mediated cancer hyperthermiaS. Paul, D. Bhatia (Eds.), Smart Healthcare for Disease Diagnosis and Prevention, Academic Press (2020), pp. 153-173
-
[29]
Magnetic nanoparticles for hyperthermia in cancer treatment: an emerging toolEnviron. Sci. Pollut. Res., 27 (16) (2020), pp. 19214-19225
-
[30]
Multi-institutional study of peritoneal sarcomatosis from uterine sarcoma treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapyEur. J. Surg. Oncol., 43 (11) (2017), pp. 2170-2177
-
[31]
Systemically delivered magnetic hyperthermia for prostate cancer treatmentPharmaceutics, 12 (11) (2020), p. 1020
-
[32]
Treatment of breast cancer-bearing BALB/c mice with magnetic hyperthermia using dendrimer functionalized iron-oxide nanoparticlesNanomaterials, 10 (11) (2020), p. 2310
-
[33]
Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancerBiomaterials, 127 (2017), pp. 25-35
-
[34]
Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticlesNat. Nanotechnol., 14 (3) (2019), pp. 260-268
-
[35]
Implication of magnetic nanoparticles in cancer detection, screening and treatmentMagnetochemistry, 5 (4) (2019), p. 55
-
[36]
Nanoparticles: properties, applications and toxicitiesArab. J. Chem., 12 (7) (2019), pp. 908-931
-
[37]
Superparamagnetic iron oxide nanoparticles—current and prospective medical applicationsMaterials, 12 (4) (2019), p. 617
-
[38]
High-sensitivity in vivo contrast for ultra-low field magnetic resonance imaging using superparamagnetic iron oxide nanoparticlesSci. Adv., 6 (29) (2020), p. eabb0998
-
[39]
Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticlesBiochem. Biophys. Rep., 13 (2018), pp. 63-72
-
[40]
Superparamagnetic iron oxides as MPI tracers: a primer and review of early applicationsAdv. Drug Deliv. Rev., 138 (2019), pp. 293-301
-
[41]
Nanoparticle based induction heating at low magnitudes of magnetic field strengths for breast cancer therapyJ. Magn. Magn. Mater., 483 (2019), pp. 169-177
-
[42]
Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug deliveryInt. J. Mol. Sci., 16 (4) (2015), pp. 8070-8101
-
[43]
Superparamagnetic iron oxide nanoparticles for cancer diagnosis and therapyJ. Biomed. Nanotechnol., 15 (2) (2019), pp. 215-416
-
[44]
Preparation and characterization of superparamagnetic iron oxide nanoparticles for magnetically guided drug deliveryInt. J. Nanomed., 13 (2018), pp. 43-46
-
[45]
Multifunctional magnetic-polymeric nanoparticles based ferrofluids for multi-modal in vitro cancer treatment using thermotherapy and chemotherapyJ. Mol. Liq., 293 (2019), Article 111549
-
[46]
Highly versatile SPION encapsulated PLGA nanoparticles as photothermal ablators of cancer cells and as multimodal imaging agentsBiomater. Sci., 5 (3) (2017), pp. 432-443
-
[47]
In-vitro study of multifunctional PLGA-SPION nanoparticles loaded with gemcitabine as radiosensitizer used in radiotherapyIran. J. Pharm. Res.: IJPR, 18 (4) (2019), pp. 1694-1703
-
[48]
Magnetic hyperthermia therapy for the treatment of glioblastoma: a review of the therapy’s history, efficacy and application in humansInt. J. Hyperth., 34 (8) (2018), pp. 1316-1328
-
[49]
Polycaprolactone-coated superparamagnetic iron oxide nanoparticles for in vitro magnetic hyperthermia therapy of cancerEur. Polym. J., 133 (2020), Article 109789
-
[50]
In vitro hyperthermia with improved colloidal stability and enhanced SAR of magnetic core/shell nanostructuresMater. Sci. Eng.: C., 59 (2016), pp. 702-709
-
[51]
HEPES-involved hydrothermal synthesis of Fe3O4 nanoparticles and their biological applicationRSC Adv., 5 (7) (2015), pp. 5059-5067
-
[52]
Antibody conjugation of nanoparticles as therapeutics for breast cancer treatmentInt. J. Mol. Sci., 21 (17) (2020), p. 6018
-
[53]
Trastuzumab conjugated porphyrin-superparamagnetic iron oxide nanoparticle: a potential PTT-MRI bimodal agent for herceptin positive breast cancerPhoto Photodyn. Ther., 31 (2020), Article 101896
-
[54]
Encapsulation of superparamagnetic iron oxide nanoparticles with polyaspartamide biopolymer for hyperthermia therapyEur. Polym. J., 122 (2020), Article 109396
-
[55]
A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapyNat. Med., 18 (2) (2012), pp. 307-314
-
[56]
Inhibition of furin by bone targeting superparamagnetic iron oxide nanoparticles alleviated breast cancer bone metastasisBioact. Mater., 6 (3) (2021), pp. 712-720
-
[57]
Multifunctional mesoporous silica nanoparticles with thermal‐responsive gatekeeper for NIR light‐triggered chemo/photothermal‐therapySmall, 12 (31) (2016), pp. 4286-4298
-
[58]
Dual enzymatic reaction-assisted gemcitabine delivery systems for programmed pancreatic cancer therapyACS Nano, 11 (2) (2017), pp. 1281-1291
-
[59]
Helix stabilized, thermostable, and protease-resistant self-assembled peptide nanostructures as potential inhibitors of protein–protein interactionsBiomacromolecules, 14 (8) (2013), pp. 2684-2689
-
[60]
Managing unwanted immunogenicity of biologicalsAutoimmun. Rev., 14 (7) (2015), pp. 569-574
-
[61]
Peptide–nanoparticle conjugates: a next generation of diagnostic and therapeutic platforms?Nano Converg., 5 (1) (2018), pp. 1-18
-
[62]
Synthesis of folic acid functionalized terbium-doped dendritic fibrous nano-silica and Interaction with HEK 293 normal, MDA breast cancer and HT 29 colon cancer cellsJ. Mol. Recognit., 33 (11) (2020), p. 2871
-
[63]
Highly sensitive and specific cytosensing of HT 29 colorectal cancer cells using folic acid functionalized-KCC-1 nanoparticlesBiosens. Bioelectron., 132 (2019), pp. 122-131
-
[64]
A facile route to hollow nanospheres of mesoporous silica with tunable sizeChem. Commun., 23 (2008), pp. 2629-2631
-
[65]
Hollow/rattle-type mesoporous nanostructures by a structural difference-based selective etching strategyACS Nano, 4 (1) (2010), pp. 529-539
-
[66]
Hollow micro‐/nanostructures: synthesis and applicationsAdv. Mater., 20 (21) (2008), pp. 3987-4019
-
[67]
Magnetic iron oxide nanoparticle-hollow mesoporous silica Spheres: Fabrication and potential application in drug deliveryCurr. Appl. Phys., 20 (2) (2020), pp. 320-325
-
[68]
Preparation and characterization of multifunctional mesoporous silica nanoparticles for dual magnetic resonance and fluorescence imaging in targeted cancer therapyMicroporous Mesoporous Mater., 250 (2017), pp. 210-220
-
[69]
Cell-induced intracellular controlled release of membrane impermeable cysteine from a mesoporous silica nanoparticle-based drug delivery systemChem. Commun., 22 (2009), pp. 3219-3221
-
[70]
R.W. Rand, H.D. Snow, D.G. Elliott, G.M. Haskins, Induction heating method for use in causing necrosis of neoplasm, Google Patents, 1985.
-
[71]
Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: a novel concept of “heat-controlled necrosis” with heat shock protein expressionCancer Immunol. Immunother., 55 (3) (2006), pp. 320-328
-
[72]
Magnetic nanomaterials for hyperthermia-based therapy and controlled drug deliveryAdv. Drug Deliv. Rev., 63 (9) (2011), pp. 789-808
-
[73]
A biodegradable MnSiO3@ Fe3O4 nanoplatform for dual-mode magnetic resonance imaging guided combinatorial cancer therapyBiomaterials, 194 (2019), pp. 151-160
-
[74]
Magnetic nanoparticles modified-porous scaffolds for bone regeneration and photothermal therapy against tumorsNanomed.: Nanotechnol. Biol. Med., 14 (3) (2018), pp. 811-822
-
[75]
Preparation of Fe3O4@ SiO2@ Tannic acid double core-shell magnetic nanoparticles via the Ugi multicomponent reaction strategy as a pH-responsive co-delivery of doxorubicin and methotrexateMater. Chem. Phys., 247 (2020), Article 122857
-
[76]
Tumor-targetable magnetoluminescent silica nanoparticles for bimodal time-gated luminescence/magnetic resonance imaging of cancer cells in vitro and in vivoTalanta, 220 (2020), Article 121378
-
[77]
SiO2-coated magnetic nano-Fe3O4 photosensitizer for synergistic tumour-targeted chemo-photothermal therapyColloids Surf. B: Biointerfaces, 195 (2020), Article 111274
-
[78]
Release of photoactivatable drugs from plasmonic nanoparticles for targeted cancer therapyACS Nano, 5 (10) (2011), pp. 7796-7804
-
[79]
Experimental and theoretical studies of light-to-heat conversion and collective heating effects in metal nanoparticle solutionsNano Lett., 9 (3) (2009), pp. 1139-1146
-
[80]
A facile single-step synthesis of ternary multicore magneto-plasmonic nanoparticlesNanoscale, 6 (7) (2014), pp. 3532-3535
-
[81]
Recent progress in syntheses and applications of dumbbell‐like nanoparticlesAdv. Mater., 21 (30) (2009), pp. 3045-3052
-
[82]
Dumbbell-like Au− Fe3O4 nanoparticles for target-specific platin deliveryJ. Am. Chem. Soc., 131 (12) (2009), pp. 4216-4217
-
[84]
Photo-controlled aptamers delivery by dual surface gold-magnetic nanoparticles for targeted cancer therapyMater. Sci. Eng.: C., 80 (2017), pp. 88-92
-
[85]
RGD-conjugated dendrimer-modified gold nanorods for in vivo tumor targeting and photothermal therapyMol. Pharm., 7 (1) (2010), pp. 94-104
-
[86]
Theranostics: combining imaging and therapyBioconjugate Chem., 22 (10) (2011), pp. 1879-1903
-
[87]
Multifunctional nanoparticles for multimodal imaging and theragnosisChem. Soc. Rev., 41 (7) (2012), pp. 2656-2672
-
[88]
A chitosan hydrogel delivery system for osteosarcoma gene therapy with pigment epithelium-derived factor combined with chemotherapyBiomaterials, 30 (27) (2009), pp. 4815-4823
-
[89]
Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermiaAdv. Mater., 20 (20) (2008), pp. 3832-3838
-
[90]
Graphene quantum dots‐capped magnetic mesoporous silica nanoparticles as a multifunctional platform for controlled drug delivery, magnetic hyperthermia, and photothermal therapySmall, 13 (2) (2017), p. 1602225
-
[91]
Coadministration of iRGD with multistage responsive nanoparticles enhanced tumor targeting and penetration abilities for breast cancer therapyACS Appl. Mater. Interfaces, 10 (26) (2018), pp. 22571-22579
-
[92]
Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapyBiomaterials, 168 (2018), pp. 64-75
-
[93]
Can magneto-plasmonic nanohybrids efficiently combine photothermia with magnetic hyperthermia?Nanoscale, 7 (45) (2015), pp. 18872-18877
-
[94]
Photothermally enhanced drug delivery by ultrasmall multifunctional FeCo/graphitic shell nanocrystalsACS Nano, 5 (2) (2011), pp. 1505-1512
-
[95]
Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxideBiomaterials, 32 (33) (2011), pp. 8555-8561
-
[96]
Multifunctional magnetic-gold nanoparticles for efficient combined targeted drug delivery and interstitial photothermal therapyInt. J. Pharm., 554 (2019), pp. 256-263
-
[97]
Multifunctional theranostic red blood cells for magnetic‐field‐enhanced in vivo combination therapy of cancerAdv. Mater., 26 (28) (2014), pp. 4794-4802
-
[98]
Therapeutic applications of CRISPR/Cas9 in breast cancer and delivery potential of gold nanomaterialsNanobiomedicine, 7 (1849543520983196) (2020)
-
[99]
Mitochondria-targeted magnetic gold nanoheterostructure for multi-modal imaging guided photothermal and photodynamic therapy of triple-negative breast cancerChem. Eng. J., 403 (2021), Article 126364
-
[100]
Polysaccharide-based nanoparticles for theranostic nanomedicineAdv. Drug Deliv. Rev., 99 (2016), pp. 70-84
-
[101]
Selective uptake of chitosan polymeric micelles by circulating monocytes for enhanced tumor targetingCarbohydr. Polym., 229 (2020), Article 115435
-
[102]
Chitosan-modified lipid nanodrug delivery system for the targeted and responsive treatment of ulcerative colitisCarbohydr. Polym., 230 (2020), Article 115613
-
[103]
Fabrication and study of curcumin loaded nanoparticles based on folate-chitosan for breast cancer therapy applicationCarbohydr. Polym., 168 (2017), pp. 14-21
-
[104]
Inhibition of tumor-promoting stroma to enforce subsequently targeting AT1R on tumor cells by pathological inspired micellesBiomaterials, 161 (2018), pp. 33-46
-
[105]
Polysaccharide-modified nanoparticles with intelligent CD44 receptor targeting ability for gene deliveryInt. J. Nanomed., 13 (2018), pp. 3989-4002
-
[106]
Boronate-crosslinked polysaccharide conjugates for pH-responsive and targeted drug deliveryChem. Commun., 55 (8) (2019), pp. 1164-1167
-
[107]
Magnetic core-shell hybrid nanoparticles for receptor targeted anti-cancer therapy and magnetic resonance imagingJ. Colloid Interface Sci., 486 (2017), pp. 112-120
-
[108]
Design of drug delivery systems containing artemisinin and its derivativesMolecules, 22 (2) (2017), p. 323
-
[109]
Artemisinin loaded chitosan magnetic nanoparticles for the efficient targeting to the breast cancerInt. J. Biol. Macromol., 104 (2017), pp. 1853-1859
-
[110]
A simple approach to design chitosan functionalized Fe3O4 nanoparticles for pH responsive delivery of doxorubicin for cancer therapyJ. Magn. Magn. Mater., 448 (2018), pp. 199-207
-
[111]
A novel biocompatible core-shell magnetic nanocomposite based on cross-linked chitosan hydrogels for in vitro hyperthermia of cancer therapyInt. J. Biol. Macromol., 140 (2019), pp. 407-414
-
[112]
Chitosan derived glycolipid nanoparticles for magnetic resonance imaging guided photodynamic therapy of cancerCarbohydr. Polym., 245 (2020), Article 116509
-
[113]
Nanoparticles as drug delivery systems in cancer medicine: emphasis on RNAi-containing nanoliposomesPharmaceuticals, 6 (11) (2013), pp. 1361-1380
-
[114]
Metal complexes in cancer therapy–an update from drug design perspectiveDrug Des. Dev. Ther., 11 (2017), pp. 599-616
-
[115]
Inorganic nanoparticles in cancer therapyPharm. Res., 28 (2) (2011), pp. 237-259
-
[116]
Properties and applications of colloidal nonspherical noble metal nanoparticlesAdv. Mater., 22 (16) (2010), pp. 1805-1825
-
[117]
Biological applications of gold nanoparticlesChem. Soc. Rev., 37 (9) (2008), pp. 1896-1908
-
[118]
Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticlesPhilos. Trans. R. Soc. A: Math. Phys. Eng. Sci., 368 (1915) (2010), pp. 1333-1383
-
[119]
Gold nanoparticles in delivery applicationsAdv. Drug Deliv. Rev., 60 (11) (2008), pp. 1307-1315
-
[120]
Nanocarriers shape up for long lifeNat. Nanotechnol., 2 (4) (2007), pp. 203-204
-
[121]
Understanding the photothermal conversion efficiency of gold nanocrystalsSmall, 6 (20) (2010), pp. 2272-2280
-
[122]
Nanoparticles for thermal cancer therapyJ. Biomech. Eng., 131 (7) (2009), Article 074001
-
[123]
Ordered arrangement of hydrated GdPO4 nanorods in magnetic chitosan matrix promotes tumor photothermal therapy and bone regeneration against breast cancer bone metastasesChem. Eng. J., 381 (2020), Article 122694
-
[124]
Magnetic-luminescent cerium-doped gadolinium aluminum garnet nanoparticles for simultaneous imaging and photodynamic therapy of cancer cellsJ. Colloid Interface Sci., 526 (2018), pp. 220-229
-
[125]
Disulfiram-loaded functionalized magnetic nanoparticles combined with copper and sodium nitroprusside in breast cancer cellsMater. Sci. Eng.: C., 119 (2021), Article 111452
-
[126]
Facile synthesis of biocompatible Fe3O4-based nanoparticles for pH-responsive dual-model magnetic resonance imaging-guided tumour eradication by photothermal therapyChin. J. Anal. Chem., 47 (5) (2019), pp. 678-685
-
[127]
Carbon-based nanomaterials: multifunctional materials for biomedical engineeringACS Nano, 7 (4) (2013), pp. 2891-2897
-
[128]
Carbon-based nanomaterials as an emerging platform for theranosticsMater. Horiz., 6 (3) (2019), pp. 434-469
-
[129]
Non-metallic nanomaterials in cancer theranostics: a review of silica- and carbon-based drug delivery systemsSci. Technol. Adv. Mater., 14 (4) (2013), Article 044407
-
[130]
Cancer targeting and drug delivery using carbon-based quantum dots and nanotubesMolecules, 23 (2) (2018), p. 378
-
[131]
Image-guided cancer therapy using aptamer-functionalized cross-linked magnetic-responsive Fe3O4@ carbon nanoparticlesAnal. Chim. Acta, 1056 (2019), pp. 108-116
-
[132]
Vries, Overcoming the blood–brain tumor barrier for effective glioblastoma treatmentDrug Resist. Updates, 19 (2015), pp. 1-12
-
[133]
Ag@ Fe3O4@ C nanoparticles for multi-modal imaging-guided chemo-photothermal synergistic targeting for cancer therapyAnal. Chim. Acta, 1086 (2019), pp. 122-132
-
[134]
Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomesFEBS Lett., 268 (1) (1990), pp. 235-237
-
[135]
Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignanciesClin. Cancer Res., 10 (11) (2004), pp. 3708-3716
-
[136]
Targeting tumor microenvironment with PEG-based amphiphilic nanoparticles to overcome chemoresistanceNanomed.: Nanotechnol., Biol. Med., 12 (2) (2016), pp. 269-286
-
[137]
Cross-linked magnetic nanoparticles with a biocompatible amide bond for cancer-targeted dual optical/magnetic resonance imagingColloids Surf. B: Biointerfaces, 161 (2018), pp. 183-191
-
[138]
Current trends in targeted therapy for drug-resistant infectionsAppl. Microbiol. Biotechnol., 103 (20) (2019), pp. 8301-8314
-
[139]
Antibody engineering promotes nanomedicine for cancer treatmentNanomedicine, 5 (8) (2010), pp. 1141-1145
-
[140]
The promotion of nanoparticle delivery to two populations of gastric cancer stem cells by CD133 and CD44 antibodiesBiomed. Pharmacother., 115 (2019), Article 108857
-
[141]
Superparamagnetic PLGA-iron oxide microcapsules for dual-modality US/MR imaging and high intensity focused US breast cancer ablationBiomaterials, 33 (24) (2012), pp. 5854-5864
-
[142]
Evaluation of superparamagnetic iron oxide-polymer composite microcapsules for magnetic resonance-guided high-intensity focused ultrasound cancer surgeryBMC Cancer, 14 (1) (2014), pp. 1-11
-
[143]
Synthesis of theranostic Anti-EGFR ligand conjugate iron oxide nanoparticles for magnetic resonance imaging for treatment of liver cancerJ. Drug Deliv. Sci. Technol., 55 (2020), Article 101367
-
[144]
In vivo delivery of silica nanorattle encapsulated docetaxel for liver cancer therapy with low toxicity and high efficacyACS Nano, 4 (11) (2010), pp. 6874-6882
-
[145]
Magnetic stomatocyte-like nanomotor as photosensitizer carrier for photodynamic therapy based cancer treatmentColloids Surf. B: Biointerfaces, 194 (2020), Article 111204
-
[146]
Magnetic nanoparticles coated with polyphenols for spatio-temporally controlled cancer photothermal/immunotherapyJ. Control. Release, 326 (2020), pp. 131-139
-
[147]
Solid lipid nanoparticles for parenteral drug deliveryAdv. Drug Deliv. Rev., 56 (9) (2004), pp. 1257-1272
-
[148]
Polymer conjugates as anticancer nanomedicinesNat. Rev. Cancer, 6 (9) (2006), pp. 688-701
-
[149]
Polymeric nanomedicines for image-guided drug delivery and tumor-targeted combination therapyNano Today, 5 (3) (2010), pp. 197-212
-
[150]
Polymers in drug delivery—state of the art and future trendsAdv. Eng. Mater., 13 (3) (2011), pp. B61-B87
-
[151]
‘Stealth’corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorptionColloids Surf. B: Biointerfaces, 18 (3–4) (2000), pp. 301-313
-
[152]
Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapiesJ. Funct. Biomater., 10 (1) (2019), p. 4
-
[153]
PLA-based magnetic nanoparticles armed with thermo/pH responsive polymers for combination cancer chemotherapyJ. Drug Deliv. Sci. Technol., 45 (2018), pp. 240-254
-
[154]
Carbonic anhydrase IX as an anticancer therapy target: preclinical evaluation of internalizing monoclonal antibody directed to catalytic domainCurr. Pharm. Des., 16 (29) (2010), pp. 3255-3263
-
[155]
Poly-L-lysine designed magnetic nanoparticles for combined hyperthermia, magnetic resonance imaging and cancer cell detectionJ. Magn. Magn. Mater., 475 (2019), pp. 316-326
-
[156]
A bio-inspired magnetic natural hydrogel containing gelatin and alginate as a drug delivery system for cancer chemotherapyInt. J. Biol. Macromol., 156 (2020), pp. 438-445
-
[157]
A multifunctional magnetic nanosystem based on “two strikes” effect for synergistic anticancer therapy in triple-negative breast cancerJ. Control. Release, 322 (2020), pp. 401-415
-
[158]
Multifunctional near-infrared dye-magnetic nanoparticles for bioimaging and cancer therapyCancer Lett., 390 (2017), pp. 168-175
-
[159]
Applications of bacterial magnetic nanoparticles in nanobiotechnologyJ. Nanosci. Nanotechnol., 16 (3) (2016), pp. 2164-2171
-
[160]
Bacterial magnetosome and its potential applicationMicrobiol. Res., 203 (2017), pp. 19-28
-
[161]
Magnetotactic bacteria as potential sources of bioproductsMar. Drugs, 13 (1) (2015), pp. 389-430
-
[162]
Applications of magnetosomes synthesized by magnetotactic bacteria in medicineFront. Bioeng. Biotechnol., 2 (2014), p. 5
-
[163]
Magnetically targeted photothemal cancer therapy in vivo with bacterial magnetic nanoparticlesColloids Surf. B: Biointerfaces, 172 (2018), pp. 308-314
-
[164]
Synergistic effect of gold nanoparticles and cold plasma on glioblastoma cancer therapyJ. Phys. D: Appl. Phys., 47 (33) (2014), Article 335402
-
[165]
Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivoPLoS One, 8 (11) (2013), Article e79325
-
[166]
Sterilization of bacteria, yeast, and bacterial endospores by atmospheric-pressure cold plasma using helium and oxygenJ. Microbiol., 44 (3) (2006), pp. 269-275
-
[167]
Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasmaIEEE Trans. Plasma Sci., 35 (5) (2007), pp. 1559-1566
-
[168]
A novel method of tooth whitening using cold plasma microjet driven by direct current in atmospheric-pressure airIEEE Trans. Plasma Sci., 38 (11) (2010), pp. 3143-3151
-
[169]
In vitro demonstration of cancer inhibiting properties from stratified self-organized plasma-liquid interfaceSci. Rep., 7 (1) (2017), pp. 1-11
-
[170]
Evaluation of plasma skin regeneration technology in low-energy full-facial rejuvenationArch. Dermatol., 143 (2) (2007), pp. 168-174
-
[171]
The effect of tuning cold plasma composition on glioblastoma cell viabilityPLoS One, 9 (5) (2014), Article e98652
-
[172]
Cold atmospheric plasma and iron oxide-based magnetic nanoparticles for synergetic lung cancer therapyFree Radic. Biol. Med., 130 (2019), pp. 71-81
-
[173]
Photonic/magnetic hyperthermia-synergistic nanocatalytic cancer therapy enabled by zero-valence iron nanocatalystsBiomaterials, 219 (2019), Article 119374