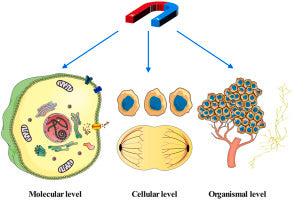
The effect of magnetic fields on tumor occurrence and progression: Recent advances
Abstract
Malignancies are the leading human health threat worldwide. Despite rapidly developing treatments, poor prognosis and outcome are still common. Magnetic fields have shown good anti-tumoral effects both in vitro and in vivo, and represent a potential non-invasive treatment; however, the specific underlying molecular mechanisms remain unclear. We here review recent studies on magnetic fields and their effect on tumors at three different levels: organismal, cellular, and molecular. At the organismal level, magnetic fields suppress tumor angiogenesis, microcirculation, and enhance the immune response. At the cellular level, magnetic fields affect tumor cell growth and biological functions by affecting cell morphology, cell membrane structure, cell cycle, and mitochondrial function. At the molecular level, magnetic fields suppress tumors by interfering with DNA synthesis, reactive oxygen species level, second messenger molecule delivery, and orientation of epidermal growth factor receptors. At present, scientific experimental evidence is still lacking; therefore, systematic studies on the biological mechanisms involved are urgently needed for the future application of magnetic fields to tumor treatment.
Graphical abstract
Summary of possible mechanisms by which magnetic fields affect tumors.

Introduction
Cancer has become a major threat to human health. The mortality rate for cancer is still very high according to the global cancer statistics of 2021 (Siegel et al., 2021), even though significant progress has been made in cancer treatment (Bray et al., 2018). Chemotherapy remains a primary choice for cancer treatment but presents many side effects. For this reason, noninvasive therapy is receiving more attention, including physiotherapy. Various types of physiotherapy have been gradually applied to tumor treatment, such as electrotherapy, photokinetic, thermal, magnetic, ultrasonic, and microwave therapies, and radiotherapy (Mantso et al., 2016; Schildkopf et al., 2010; Correia et al., 2015; Allison et al., 2011; Qu et al., 2012; Eltejaei et al., 2021; Sachsman et al., 2015; Satkauskas et al., 2005; Chen et al., 2010). Among these, magnetic therapy has received considerable attention (Zhang et al., 2017a).
Magnets have been used in the cure of disease for more than two thousand years. Recently, magnetic therapy has been gradually applied to clinical treatments, such as peripheral nerve regeneration (Suszyński et al., 2014), osteonecrosis (Ding et al., 2011), pain reduction (Zhu et al., 2017; Kiss et al., 2013; Eccles, 2005a, 2005b; Schuster and Rapoport, 2016), regulation of muscle function (Bergman et al., 2004; Chaloupka et al., 2002), anti-inflammation (Juhász et al., 2014), and promotion of wound healing (Zhao et al., 2017; Lv et al., 2021; Shang et al., 2019; Strauch et al., 2007; Jing et al., 2010). Compared with other treatments, magnetic fields have the advantage of being noninvasive, safe, highly efficient, inexpensive, and without the risk of infection or scars.
Based on the characteristics of the generated magnetic field (MF), MFs can be classified into constant magnetic field (CMF) or dynamic magnetic field (DMF). A CMF is also known as a static magnetic field (SMF) or magnetostatic field (MSF), which can be generated by permanent magnets or solenoidal coils with unidirectional currents. According to the magnetic field strength, CMFs can be classified into weak (<1 mT), medium (1 mT–1 T), strong (1–5 T), and ultra-strong (>5 T) MFs (Hunt et al., 2009; Van Huizen et al., 2019; Mild et al., 2002). The DMF varies with time, and it can be classified as an alternating magnetic field (AMF), pulsed magnetic field (PMF), pulsating magnetic field (PuMF), and geomagnetic field (GMF) according to the mode of magnetic field production. AMFs are either generated by an electromagnetic coil with a current of a certain frequency, or by a magnet with regular motion. PMFs, PuMFs, and GMFs are produced by an electromagnetic coil with a pulsed current, AC power supply, and Earth and ionosphere, respectively (Hristov and Perez, 2011; Hildebrandt et al., 2002; Jedrzejczak-Silicka et al., 2002).
Currently, research is mainly focused on MFs with fixed parameters (Dini and Abbro, 2005; Zhang et al., 2017b), including SMFs, or oscillating MFs induced by an electromagnetic coil with a single fixed orientation. Preliminary studies have shown that MFs can affect tumor cell morphology, membrane structure, cell metabolism, growth, adhesion, immune response, and microcirculation. In most studies, this domino effect was determined using a single MF parameter or a limited types of tumor cells. Zablotskii et al. found that the magnetic susceptibility of tumor cells differed owing to their different cellular contents (Zhu et al., 2017). Therefore, the biological effects of an MF on tumor cells is dependent on various MF parameters, such as intensity, frequency, and exposure duration. The type of tumor cell is also critically important, but systematic investigations are still lacking.
Although there is increasing evidence that MFs can inhibit tumor progression, the underlying mechanism is still poorly understood. Therefore, we systematically reviewed the progress in this field, and the possible biological effects of MFs on tumors were analyzed on three different levels, namely organismal, cellular, and molecular. This study provides a theoretical basis for MF suppression of tumor growth and will be helpful for the rational application of MFs in tumor treatment in the future.
Section snippets
Immune system
The occurrence and progression of tumors are closely related to the immune system. Under an MF, a large number of immune cells were found to grow near sarcomas; therefore, it was speculated that MFs might elevate the activity of immune cells and promote phagocytosis of apoptotic bodies of immune cells, thus inducing the death of tumor cells. Gong et al. showed that the activity of splenic NK cells in SP2/0 xenograft mice increased, and IL-1 of macrophages increased significantly after exposure
Cell morphology
Cell morphology is fundamental to all vital movements and varies under MFs. Dini et al. found bubbles on the U937 cell membrane, a rough cell surface resulting from the exposure to an SMF of 6 mT for 12 h (Dini et al., 2009). After 96 h of exposure to a 50 Hz EMF of either 0.23 mT or 1.32 mT, both cell number and polarization coefficient of PC12 pheochromocytoma cells were significantly increased in the 0.23 mT EMF group compared with the control, while the opposite occurred in the 1.32 mT EMF
DNA synthesis
Many studies have found that DMFs can selectively damage the DNA of tumor cells, thereby inhibiting their proliferation. Wolf et al. found that extremely low-frequency EMFs can break the DNA strands of tumor cells. Some studies have shown that 0.1–40 MHz low-power EMFs also inhibited gallbladder tumor cell proliferation by breaking DNA strands, and that the DNA damage was non-linearly correlated with the frequency of the MF. The nucleocytoplasmic ratio (N/P) is an important indicator for the
Conclusions and prospects
In summary, it is known that the biological effects of MFs on tumors occur at the organismal, cellular, and molecular levels. At the organismal level, MFs enhance the activity of immune cells, especially NK, T, and B cells. Tumor angiogenesis is also disturbed under MFs, with a decreasing density and diameter of vessels, flow rate of red blood cells, and blood flow velocity. Moreover, it significantly increased the leakage of tumor microvessels, further injuring tumor vascular endothelial
Declaration of competing interest
All authors disclosed no relevant relationships. The author declared no potential conflicts of interest with respect to the research, author-ship, and publication of this article.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant numbers 52177226, 82172063]; the Shaanxi Provincial Key R&D Program [grant number 2021 KW-62]; the Innovation Capability Support Program of Shaanxi [grant number 2020TD-042]; the China Postdoctoral Science Foundation [grant number 2019T120948]; the Shaanxi Postdoctoral Science Foundation [grant number 2018BSHEDZZ02]; the Innovation Foundation for Doctor Dissertation of Northwestern Polytechnical University [grant